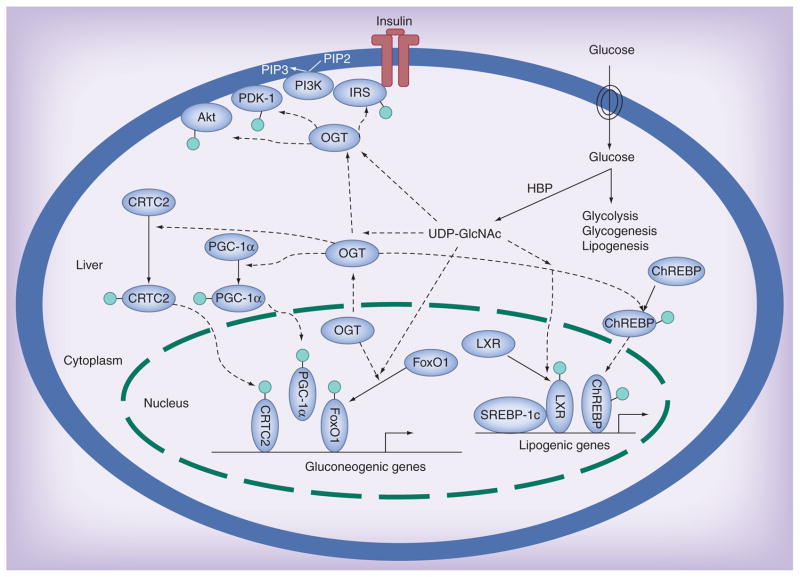
Figure 2. Protein O-GlcNAcylation attenuates insulin sensitivity.
Insulin binding to its tyrosine kinase receptor activates intracellular substrates (e.g., IRS1/2), initiating PI3K-Akt pathways and thus increased glucose uptake and metabolism in cells. PI3K activation also gives rise to PIP3, recruiting OGT to the plasma membrane, where the O-GlcNAcylation of several proximal elements leads to insulin signaling attenuation. Upon chronic hyperglycemia (insulin resistance), a rich UDP-GlcNAc pool produced from the HBP flux results in abnormally high O-GlcNAcylation of proteins (including some key transcription factors and coactivators), stimulating gluconeogenic/lipogenic gene transcription, which further diminishes insulin sensitivity (light green circle denotes the O-GlcNAc moiety).