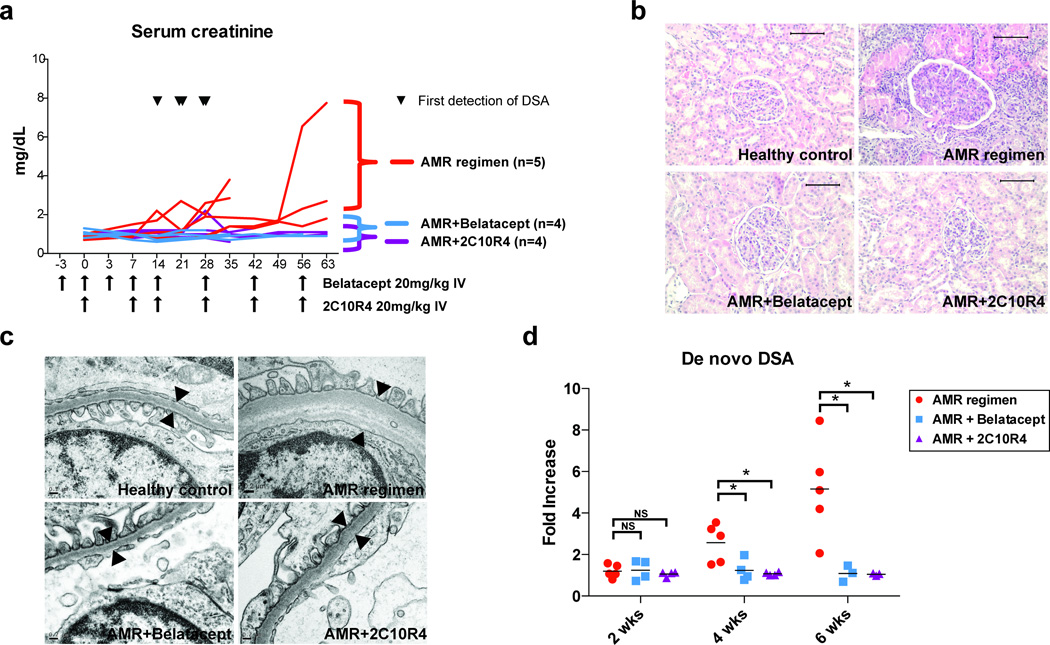
Figure 2. Costimulation blockade prevents allograft rejection and alloantibody production.
(a) Serum creatinine level of AMR positive control, belatacept-treated, and 2C10R4-treated groups. AMR positive control group (n=5, red line), receiving CD3-immunotoxin, alefacept, and tacrolimus (trough 8–12ng/mL), had early alloantibody production within 4 weeks from transplantation (the first detection of DSA from AMR positive controls is indicated by ▼) and were sacrificed for graft failure. Belatacept-treated (n=4, blue line) and 2C10R4-treated (n=4, purple line) groups received the AMR regimen plus additional belatacept or additional 2C10R4 for 8 weeks (duration and frequency indicated by arrows along the x-axis); these animals failed to demonstrate DSA production and retained graft function, but were sacrificed for non-rejection end-point criteria. Animal survival was equivalent among the three groups (p=0.632). (b) Histological examination of necropsy kidney specimens from healthy control, AMR control, belatacept-added, and 2C10R4-added group. Representative H&E staining portrays evidence of cellular and antibody-mediated rejection in the AMR positive control group, including tubulointerstitial inflammation and transplant glomerulopathy. These features were prevented in animals receiving additional costimulation blockade. Original magnification ×200 (bar scale: 100µm). (c) An electron microscopy image of glomerular basement membranes from healthy control, AMR control, Bela-added, and 2C10R4 added group. AMR control animals showed thickening, lamination and duplication of glomerular basement membranes. (d) De novo DSA production was inhibited in animals receiving belatacept and 2C10R4. *p<0.05 vs. AMR control.