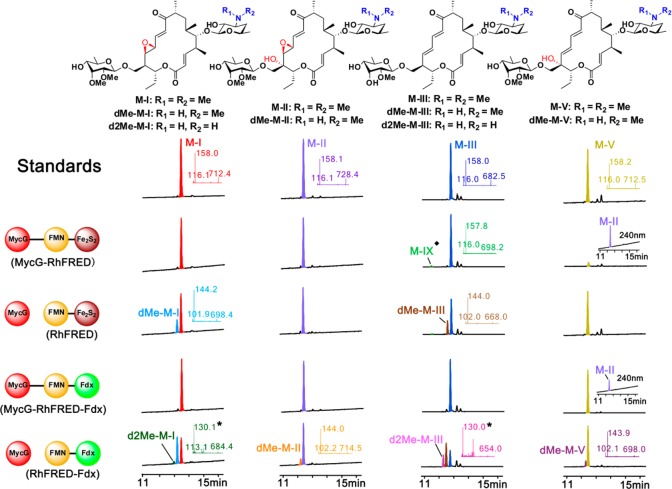
Figure 3.
LC-MS analysis of the MycG reactions using M-I, M-II, M-III, and M-V as substrates. The structures of substrates and products are shown on top. The reactions catalyzed by the same enzyme(s) are aligned in each row. The reactions using the common substrate are arranged in the same column. The LC traces of M-I and M-II were recorded at 240 nm. The LC traces of M-III and M-V were recorded at 280 nm. In the M-V reactions catalyzed by two fusion MycG enzymes, the accumulation of M-II (seen at 240 nm) that is coeluted with the unreacted M-V is shown in insets. The selected MS/MS results are displayed in insets, whose colors are consistent with those of the corresponding LC peaks. The asterisked numbers indicate the mass for the secondary ion fragment of double-demethylated mycinamicins (Figure S1). The structure of M-IX (the hydroxylated M-III labeled by the diamond symbol) is shown in Figure S8.