Figure 5.
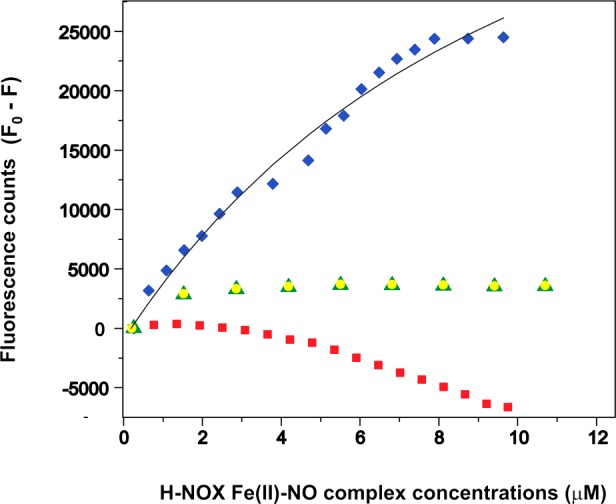
Tryptophan fluorescence quenching experiments for assessing SwH-NOX/SwHaCE binding. The Fe(II)–NO complex of SwH-NOX [WT (blue diamonds), E16K (red squares), F17A (green triangles), and E20K (yellow circles)] was titrated into a SwHaCE solution (1 μM) to determine the amount of SwHaCE Trp quenching. The difference in fluorescence (F0 – F) with and without SwH-NOX is plotted as a function of SwH-NOX Fe(II)–NO complex concentration. WT SwH-NOX caused significant quenching of SwHaCE fluorescence. In general, the SwH-NOX mutants do not quench SwHaCE fluorescence, indicating a loss of binding. From these data, the apparent equilibrium dissociation constant for the SwH-NOX/SwHaCE complex was determined to be ∼2.5 μM (the gray line shows the fit used to determine KD,app). Each titration was conducted in triplicate.
