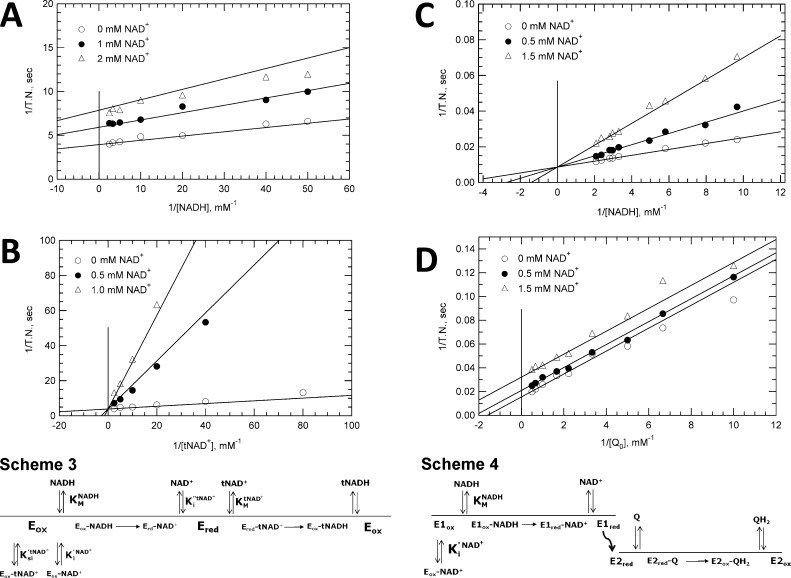
Figure 4.
Kinetics of NAD+ inhibition for NADH-tNAD+ transhydrogenase and NADH-UQ0 reductase activities. Data are presented in double-reciprocal plot form. Lines through data were generated using kinetic and inhibition constants from global fits of the original velocity vs substrate data with assumptions as described in the text. (A) Inhibition of transhydrogenase activity by NAD+ with NADH as the varied substrate. Reaction mixtures contained 25 nM NDH-2 and 1.0 mM tNAD+ as the fixed substrate with 0.0 mM NAD+ (○), 1.0 mM NAD+ (●), or 2.0 mM NAD+ (△). Fitting of data to obtain Ki values assumed noncompetitive inhibition. (B) Inhibition of transhydrogenase activity by NAD+ with tNAD+ as the varied substrate. Reaction mixtures contained 25 nM NDH-2 and 0.2 mM NADH with 0.0 mM NAD+ (○), 0.2 mM NAD+ (●), or 1.0 mM NAD+ (△). Data were fit assuming competitive inhibition. (C) NAD+ inhibition of NADH-UQ0 activity with NADH as the variable substrate and UQ0 at a fixed concentration. Reaction mixtures contained 5.0 nM NDH-2 and 2.0 mM UQ0 with 0.0 mM NAD+ (○), 0.5 mM NAD+ (●), or 1.5 mM NAD+ (△). Data were fit globally assuming uncompetitive inhibition. (D) Inhibition of NAD+ on NADH-UQ0 reductase activity with UQ0 as a variable substrate and NADH at a fixed concentration. Reaction mixtures contained 5.0 nM NDH-2, buffer, and 0.125 mM NADH with 0.0 mM NAD+ (○), 0.5 mM NAD+ (●), or 1.5 mM NAD+ (△). Data were fit assuming competitive inhibition.