Figure 1. Reaction of the protein C pathway.
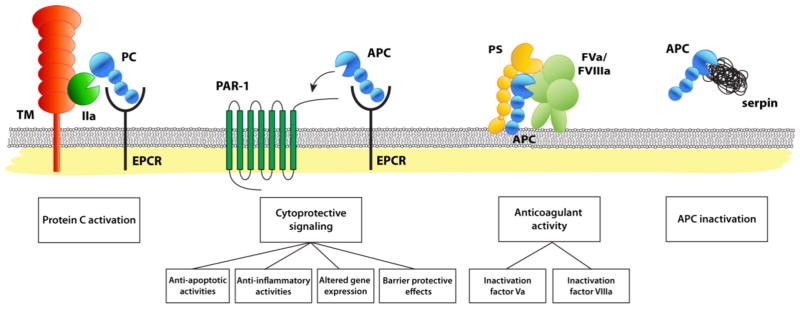
Schematic representation of the protein C pathway reactions, from left to right: protein C activation, the cytoprotective protein C pathway, the anticoagulant protein C pathway and the inactivation of APC by serine protease inhibitors (SERPINs). Protein C activation: physiologic activation of protein C (PC) by the thrombin (IIa)-thrombomodulin (TM) complex occurs on the surface of endothelial cell membranes when protein C is bound to the endothelial receptor (EPCR). Since protein C and APC have a similar affinity for EPCR, after activation APC can remain bound to EPCR to initiate cytoprotective signaling. The cytoprotective protein C pathway: APC’s direct effects of on endothelial cells require the cellular receptors EPCR and PAR1. These cellular activities of APC include anti-apoptotic and anti-inflammatory activities, alteration of gene expression profiles, and protection of endothelial barrier functions and are collectively referred to as APC’s cytoprotective activities. The anticoagulant protein C pathway: APC anticoagulant activities involve proteolytic cleavages of FVa and FVIIIa. Different protein cofactors, such as protein S (PS), FV, and various lipid cofactors (e.g. phosphatidylserine, phosphatidylethanolamine cardiolipin, glucosylceramide, etc.), enhance the inactivation of FVa and FVIIIa by APC. APC inactivation: Inactivation of APC in plasma by serine protease inhibitors (SERPINs) is slow, which contributes to a remarkably long circulation half-life of APC (~ 20 min). Most important inhibitors of APC in plasma are protein C inhibitor (PCI), plasminogen activator inhibitor-1 (PAI-1), and α1-antitrypsin and, to a lesser extent, α2-macroglobulin and α2-antiplasmin.
