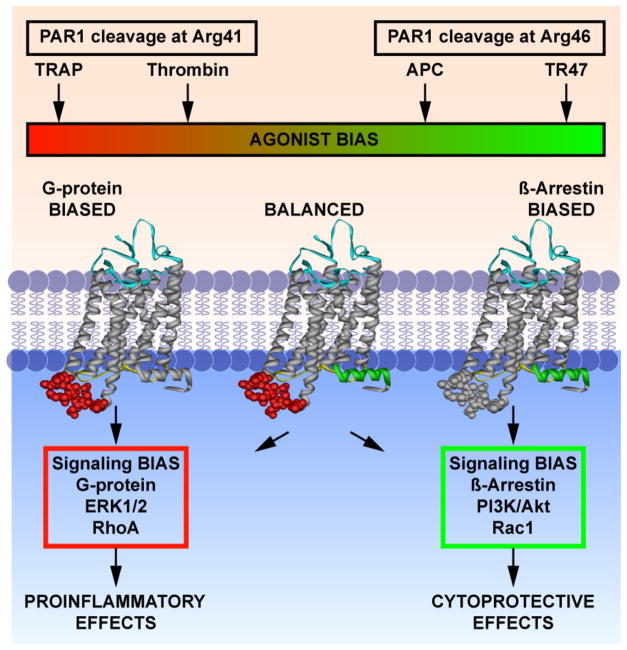
Figure 3. Induction of biased signaling by canonical and non-canonical activation of PAR1.
Schematic representation of the fundamental and functional differences between APC and thrombin-mediated PAR1 activation. APC cleavage of PAR1 at Arg46 and the tethered-ligand sequence generated by this cleavage starting at Asn47 (TR47) induces a subset of PAR1 conformations that prefer signaling mediated by β-arrestin-2 involving activation of the PI3K-Akt pathway and that result in activation of barrier protective Rac1. In contrast, thrombin cleavage of PAR1 at Arg46 and the tethered-ligand sequence generated by this cleavage starting at Ser42 (TRAP) induces a subset of PAR1 conformations that prefer G protein-mediated signaling involving activation of the ERK1/2 and that result in activation of barrier disruptive RhoA. Thus, depending on the activating ligand, PAR1 can recruit different signaling pathways that result in different functional outcomes, which has been labeled “biased agonism.” The agonist bias is thus directly related to the cleavage sites of the tethered-ligand and the new N-terminal sequence as represented by the TRAP peptide that exists after cleavage at Arg41 or the TR47 peptide that exists after cleavage at Arg46. This figure was originally published in Blood.89 L.O. Mosnier, R.K. Sinha, L. Burnier, E.A. Bouwens, J.H. Griffin. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 2012;120:5237-46. © the American Society of Hematology.