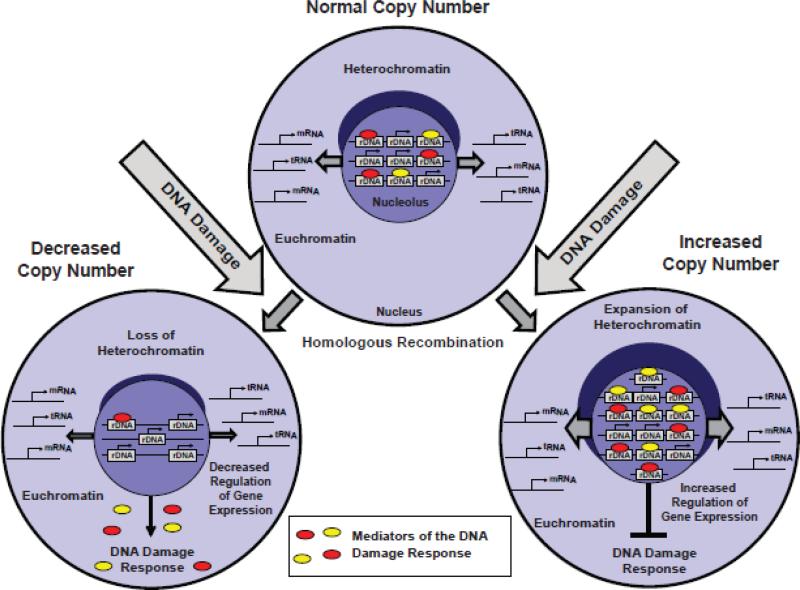
Figure 7. A hypothetical model summarizing possible causes and consequences of rDNA instability.
The DNA damage-stimulated homologous recombination (HR) is a likely cause of rDNA instability in the degenerating brain. The moderate changes in rDNA copy number as those reported here are not expected to significantly affect ribosomal biogenesis. Instead, ribosomal biogenesis-independent functions of rDNA may be affected including control of the DNA damage response (DDR), maintenance of heterochromatin, and expression regulation of the non-rRNA genes that are located in the euchromatin. One can speculate that higher content of rDNA may support cell survival by suppressing the DDR, stabilizing chromatin and stronger regulatory control of the euchromatic genes. Conversely, lower rDNA content may have opposite effects on cell sensitivity to DNA damage as well as other forms of stress by enhancing the DDR, reducing control over euchromatin gene expression and destabilizing the chromatin architecture. Thus, rDNA amplification in the degenerating regions of the DLB- or AD brain may be a consequence of increased genotoxic stress. In turn, cells with the higher genomic content of rDNA may become overrepresented due to lower sensitivity to subsequent injuries.