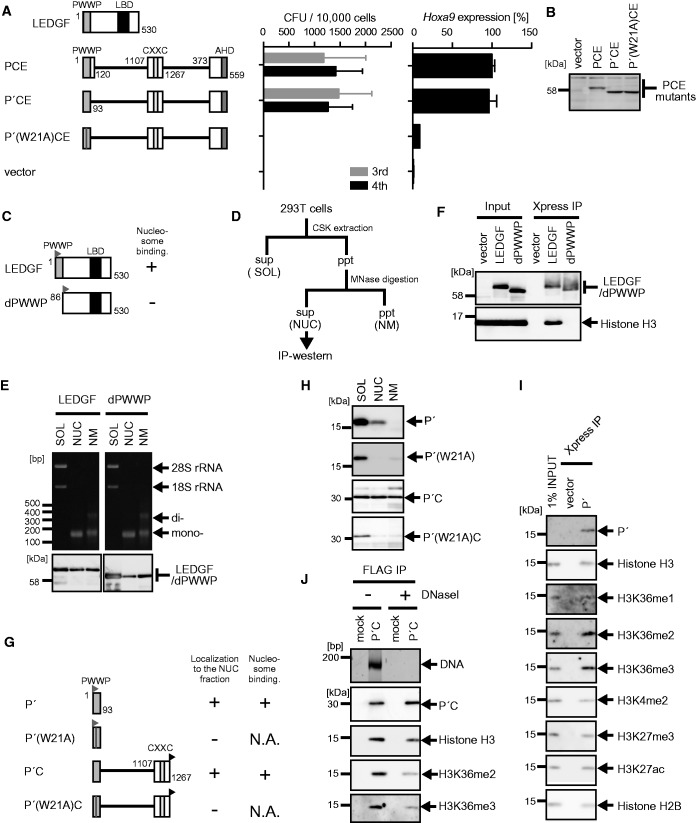
Figure 2.
Nucleosome binding by the PWWP domain is essential for MLL-ENL-dependent transformation. (A) Transforming ability of PCE mutants containing various mutations within the PWWP domain. P′: the minimum structure of the PWWP domain. The schematic structures of LEDGF and various PCE mutants are shown (left). The CFUs at the third and fourth rounds of replating are shown with error bars (SD of >3 independent experiments) (middle). Hoxa9 expression in the first-round colonies is expressed relative to that of PCE (arbitrarily set at 100%) with error bars (SD of triplicate PCRs) (right). (B) Protein expression of the PCE mutants in the packaging cells. The PCE mutant proteins were visualized using the anti-ENL antibody. (C) The schematic structures of LEDGF and its mutant lacking the PWWP domain (dPWWP). An Xpress tag (gray flag) is fused to the N-terminal end of the LEDGF proteins. Their ability to associate with nucleosomes is summarized. (D) Experimental scheme of subcellular fractionation. (E) Distribution of DNAs/RNAs (top) and LEDGF proteins (bottom) in the three subfractions. 293T cells transiently expressing the indicated proteins were subfractionated into the soluble fraction (SOL), the nucleosome fraction (NUC) and the nuclear matrix fraction (NM). The LEDGF proteins were visualized using anti-Xpress antibody. (F) Nucleosomes associated with LEDGF and dPWWP. Nucleosome co-IP assay was performed with the nucleosome fractions of 293T cells transiently expressing the LEDGF proteins. The LEDGF mutant proteins and histone H3 proteins were visualized using anti-Xpress and histone H3 antibodies, respectively. (G) The schematic structures of the P′ and the P′C mutants. An Xpress tag (gray flag) is fused to the N-terminal end of P′ mutants. A FLAG tag (black flag) is fused to the C-terminal end of the P′C mutants. The localization to the nucleosome fraction of each mutant and its ability to bind nucleosomes are summarized. N.A., not applicable. (H) Subcellular distribution of the P′ and P′C mutants. Experiments were performed as in (E). The P′ mutants were visualized by using the anti-Xpress antibody. The P′C mutants were visualized by using the anti-FLAG antibody. (I) Nucleosomes associated with P′. P′ and its associating nucleosomes were co-purified by co-IP from the NUC fraction of 293T cells transiently expressing P′ as in (F). Nucleosomes were visualized by antibodies specific for mono-methylated histone H3 lysine 36 (H3K36me1), H3K36me2 and H3K36me3 or by anti-histone H2B and -H3 antibodies. Di-methylated histone H3 lysine 4 (H3K4me2), tri-methylated histone H3 lysine 27 (H3K27me3) and acetylated histone H3 lysine 27 (H3K27ac) were also analyzed for comparison. (J) The role of DNAs in P′C–nucleosome interaction. P′C and its associating nucleosomes were co-purified by co-IP using the anti-FLAG antibody from the NUC fraction of 293T cells expressing P′C. The precipitates were treated with or without DNase I (300 kU/ml) for 10 min and washed to remove unbound material. DNAs co-precipitated with P′C were visualized by SYBR Green staining. Nucleosomes were visualized by antibodies specific for H3K36me2 and H3K36me3 modification or by the anti-histone-H3 antibody.