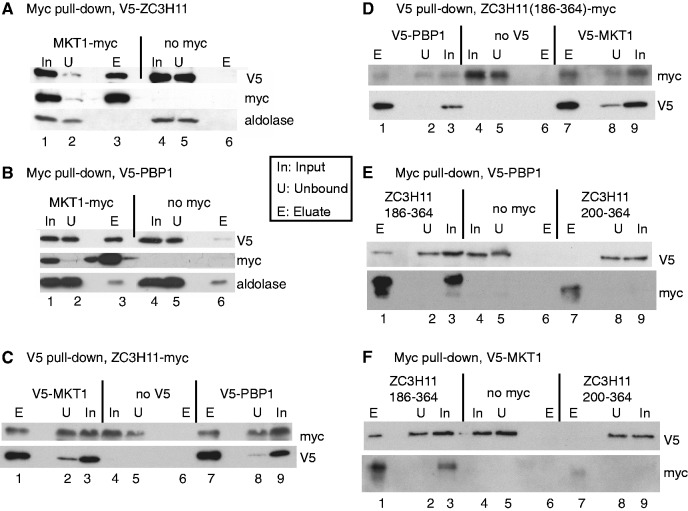
Figure 1.
Interactions of ZC3H11, MKT1 and PBP1 in trypanosomes. (A) ZC3H11 interacts with MKT1. Extracts from cells expressing V5-ZC3H11, with (lanes 1–3) or without (lanes 4–6) additional expression of MKT1-myc, were subjected to immunoprecipitation with anti-myc. The precipitated proteins were analysed by western blotting, using anti-myc, anti-V5 and, as control, anti-aldolase. In: input, U: unbound (2 × 106 cell-equivalents), E: eluate (3.8 × 107 cell-equivalents). In all cases cells were pre-incubated with MG132 for an hour prior to lysis to facilitate detection of the ZC3H11. This panel is for procyclic forms but all others are for bloodstream forms. (B) MKT1 interacts with PBP1. As in (A), but here the cells express V5-PBP1 with or without MKT1-myc. (C). ZC3H11 interacts with PBP1 and MKT1. Cells expressed ZC3H11-myc without (lanes 4–6) or with either V5-PBP1 (lanes 7–9) or V5-MKT1 (lanes 1–3). The pull-down was with anti-V5. (D) The C-terminal half of ZC3H11, ZC3H11(186-364), is pulled down by immunoprecipitation of PBP1 and MKT1. Cells expressed ZC3H11(186-364)-myc. Other details are as in (C). (E) PBP1 is pulled down by immunoprecipitation of the C-terminal half of ZC3H11, ZC3H11(186-364). Cells expressed V5-PBP1 either without myc-tagged protein (lanes 4–6), or with ZC3H11(186-364)-myc (lanes 1–3) or ZC3H11(200-364)-myc (lanes 7–9). Pull-down was with anti-myc. ZC3H11(200-364)-myc was too poorly expressed to draw conclusions. (F) MKT1 is pulled down by immunoprecipitation of the C-terminal half of ZC3H11, ZC3H11(186-364). Experiment is as for (F), except that the V5-tagged protein was MKT1.