Figure 2.
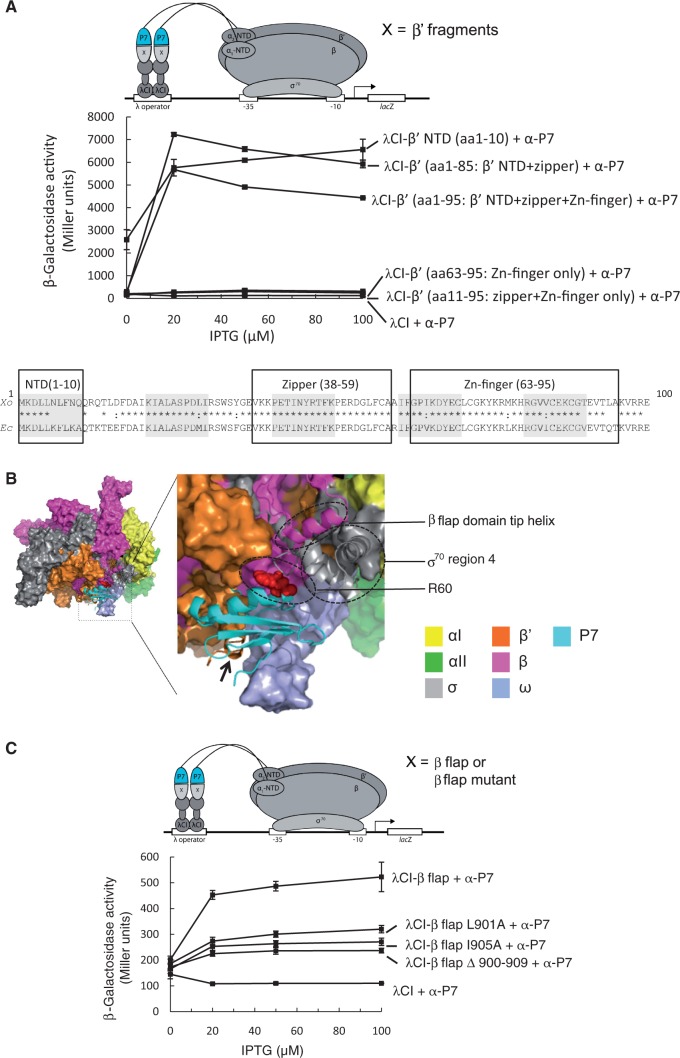
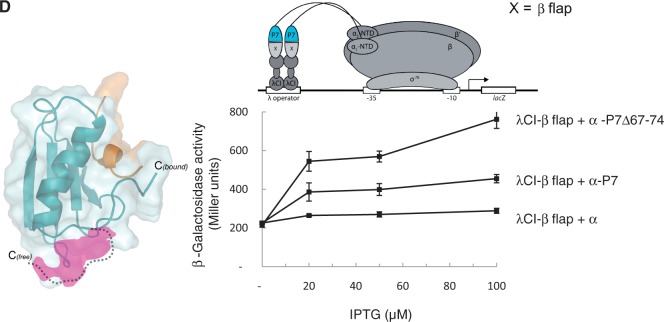
P7 interacts with the β subunit flap domain of the RNAp. (A) BTH interaction assay used to detect protein–protein interaction between different fragments of the Xo β’ subunit (as indicated and see text for details) and P7. The diagram depicts how the interaction between different fragments of the Xo β’ subunit, fused to the bacteriophage λ CI protein (λCI), and P7, fused to the α-NTD (α-P7), activates transcription of the lacZ gene. Because the expression of the fusion proteins are under the control of IPTG-inducible promoters, and the cells used for the β-galactosidase assays were grown in the presence of increasing concentrations of IPTG, in the graph the β-galactosidase activity (in Miller units) is expressed as a function of IPTG concentration. The alignment of the aa sequence corresponding to residues 1–100 of the β’ subunit of Ec and Xo RNAp is shown for comparison with the β’ NTD, zipper and Zn-finger domains boxed. In the alignment, ‘*’ and ‘:’ indicate identical and similar aa residues, respectively. (B) Surface representation of the structural model of P7 bound to the Ec Eσ70. The αI, αII, β, β’, ω and σ70 subunits coloured as indicated. The boxed region is enlarged and shows the region around the P7-RNAp interface in more detail. The σ70 region 4 and the β flap domain tip helix are shown as cartoon representation and circled, and the black arrow points to the β’ NTD; the side-chain of aa residue R60 of P7 is shown as red surface representation and circled. (C) As in (A) but the BTH interaction assay used to detect protein–protein interaction between the Ec β flap domain (and mutants thereof, as indicated) and P7. (D) Left. Surface representation of free and bound forms of P7 showing the conformational changes in the C-terminus ‘tail’ of P7. The region in P7 unveiled on binding to the β’ NTD is shown in pink. Right. As in (A), but BTH interaction assay is used to detect protein–protein interaction between the Ec β flap domain and P7 and a truncated P7 mutant lacking the C-terminal ‘tail’ (P7Δ67–74).