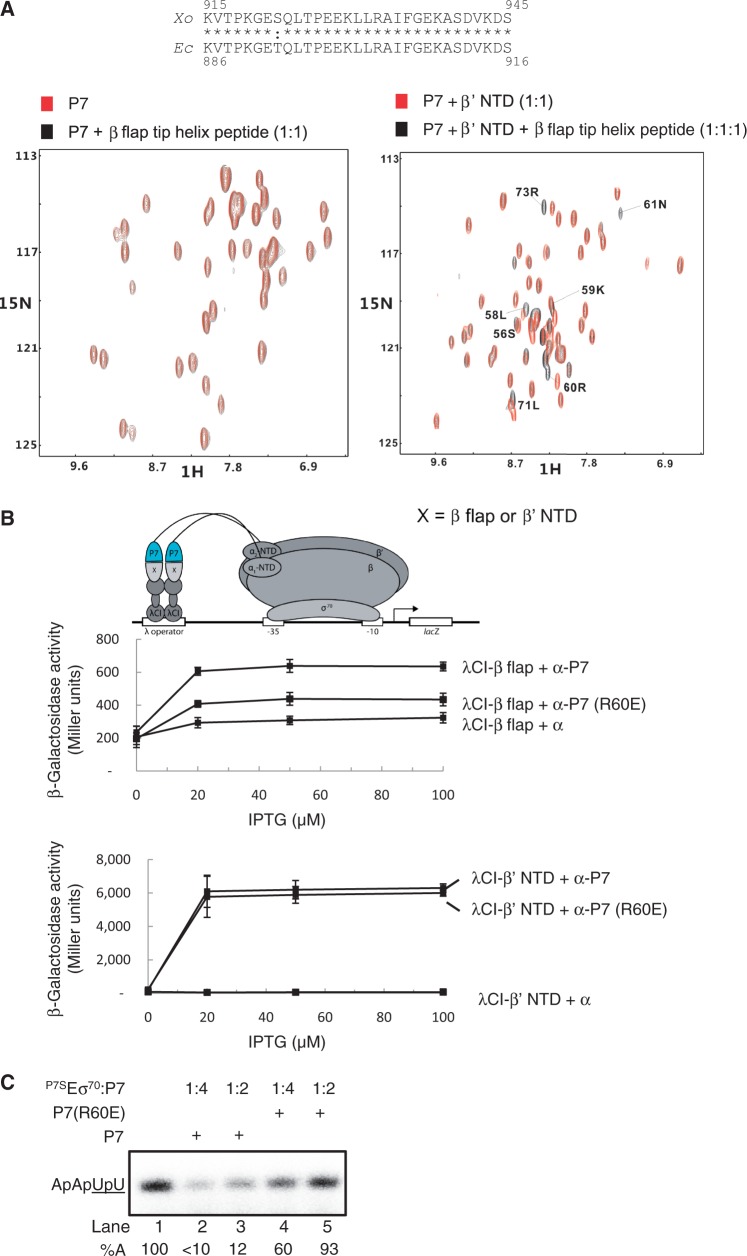
Figure 3.
P7-β flap interaction is important for transcription inhibition. (A) 1H-15N-HSQC NMR spectra of either 15N,13C-labelled P7 (left) or the p7- β’ NTD complex (right) in the presence and absence of the β flap domain tip helix peptide based on Xo β flap domain aa sequence. The aa residues significantly affected in P7 are labelled. The alignment of the aa sequence corresponding to the β flap domain tip helix from the Ec and Xo β subunit is shown for comparison. In the alignment, ‘asterisk’ and ‘colon’ indicate identical and similar aa residues, respectively. (B) BTH interaction assay used to detect protein–protein interaction between the β’ NTD or Ec β flap domain and P7 and P7 (R60E) mutant. The diagram depicts how the interaction between the β’ NTD or Ec β flap domain, fused to the bacteriophage λ CI protein (λCI), and P7 and P7 (R60E), fused to the α-NTD (α-P7), activates transcription of the lacZ gene. Because the expression of the fusion proteins (λCI-β’ NTD and flap λCI-β flap and α-P7) are under the control of IPTG-inducible promoters, and the cells used for the β-galactosidase assays were grown in the presence of increasing concentrations of IPTG, in the graphs the β-galactosidase activities (in Miller units) are expressed as a function of IPTG concentration. (C) Autoradiograph of 20% (w/v) denaturing urea gel showing the synthesis of ApApUpU transcript (underlined nucleotides are α32P labeled) from the lacUV5 promoter by P7SEσ70 in the absence (lane 1) and presence of P7 (lanes 2 and 3) and P7 (R60E) (lanes 4 and 5). The percentage of ApApUpU transcript synthesized (%A) in the reactions with P7 with respect to reactions without P7 is normalized to unincorporated [α-32P]-UTP (not shown) and given at the bottom of the gel for each reaction. All values for %A obtained from at least three independent experiments fell within 5% of the value shown.