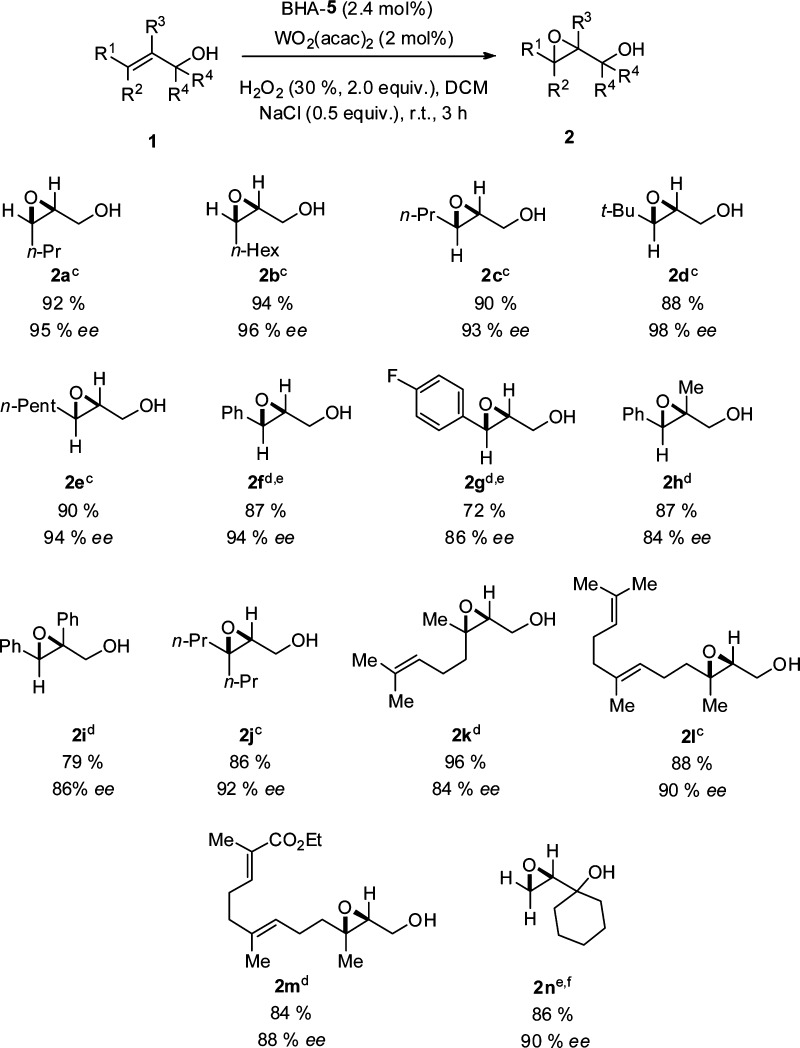
Chart 1. Asymmetric Epoxidation of Allylic Alcoholsa,b,c,d,e,f,8.
a Unless otherwise stated, reactions were performed on a 0.50 mmol scale of allylic alcohols 1 using 2.0 equiv; 30% aqueous H2O2, 2 mol % WO2(acac)2, 2.4 mol % BHA ligand, and 0.5 equiv NaCl at rt in 5 mL DCM.
b Yields of the isolated products.
c Determined by HPLC on a chiral stationary phase on the corresponding benzoates.
d Determined by HPLC on a chiral stationary phase.
e 5 mol % WO2(acac)2 and 5.5 mol % BHA-5 were used. Reaction time: 24 h
f Determined by GC on a chiral stationary phase.