Table 1. Ligand, Additive, and Solvent Screening for the Asymmetric Epoxidationa.
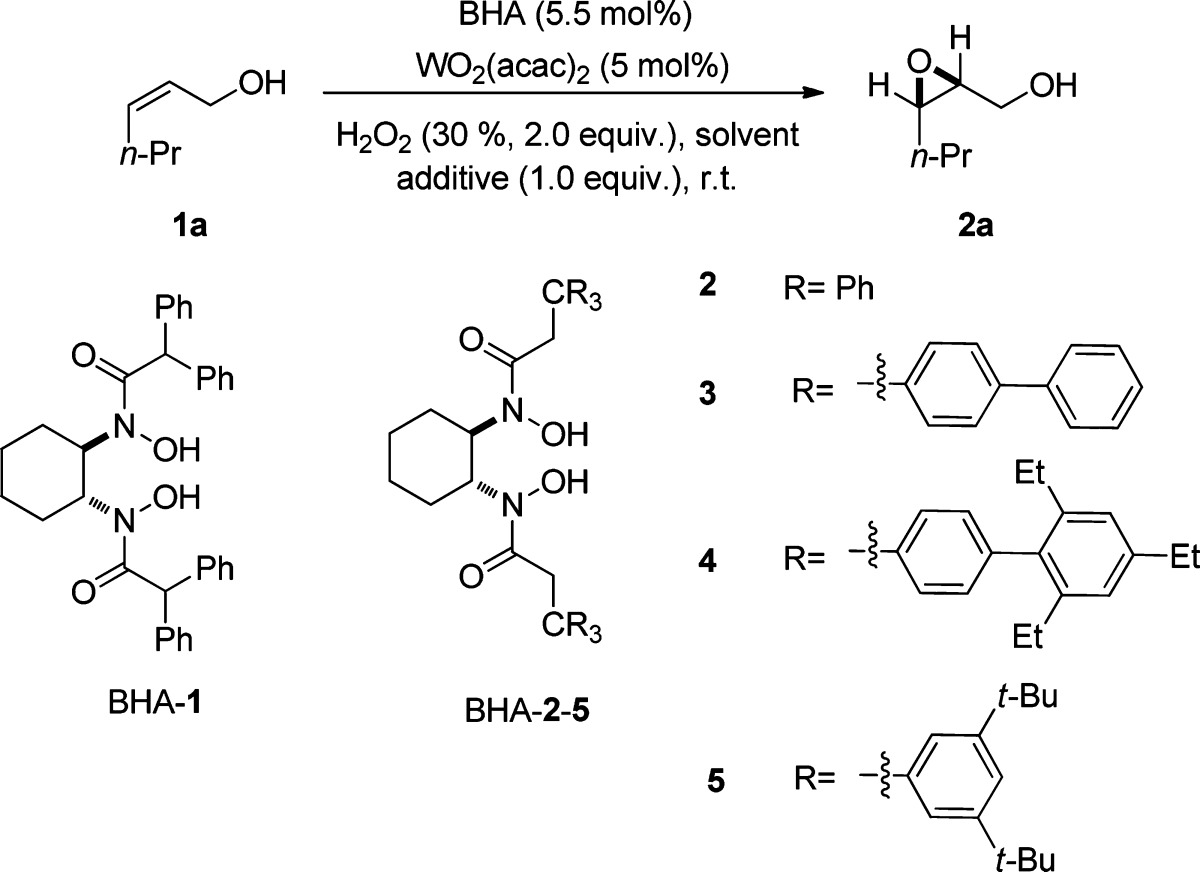
| entry | ligand | solvent | additive | t (h) | yield (%)b | ee (%)c |
|---|---|---|---|---|---|---|
| 1 | 1 | DCM | – | 2.5 | 38 | –12 |
| 2 | 2 | DCM | – | 2.5 | 54 | 37 |
| 3 | 3 | DCM | – | 2.5 | 69 | 49 |
| 4 | 4 | DCM | – | 2.5 | 71 | 41 |
| 5 | 5 | DCM | – | 2.5 | 84 | 94 |
| 6 | d | DCM | – | 8 | traces | n.d.e |
| 7 | 5 | DCM | LiCl | 24 | 81 | 93 |
| 8 | 5 | DCM | LiBr | 24 | 0 | – |
| 9 | 5 | DCM | NaCl | 2.5 | 91 | 96 |
| 10 | 5 | DCM | LiF | 2.5 | 92 | 95 |
| 11 | 5 | DCM | Na2SO4 | 2.5 | 92 | 95 |
| 12 | 5 | toluene | NaCl | 24 | 83 | 93 |
| 13 | 5 | THF | NaCl | 24 | 49 | 55 |
| 14f | 5 | DCM | NaCl | 24 | 51 | 92 |
| 15g | 5 | DCM | NaCl | 24 | 73 | 94 |
| 16g,h | 5 | DCM | NaCl | 8 | 87 | 95 |
| 17g,h,i | 5 | DCM | NaCl | 3 | 92 | 95 |
| 18h,j | 5 | DCM | NaCl | 24 | 62 | n.d.e |
| 19j,k | 5 | DCM | NaCl | 24 | 86 | 79 |
| 20g,h,i,l | 5 | DCM | NaCl | 3 | 89 | 96 |
Unless otherwise stated, reactions were performed on a 0.25 mmol scale of cis-2-hexen-1-ol (1a) using 2.0 equiv; 30% aqueous H2O2, 5 mol % WO2(acac)2, 5.5 mol % BHA ligand, and 1.0 equiv additive at rt in 5.0 mL solvent.
Yields of isolated products.
Determined by HPLC on a chiral stationary phase on the corresponding benzoate.
(−)-TADDOL was used as ligand.
Not determined.
WO2Cl2 was used instead of WO2(acac)2.
2.0 mol % WO2(acac)2 and 2.4 mol % BHA-5 were used.
0.5 equiv NaCl was used.
Reaction was performed in 2.5 mL DCM.
Reactions were performed on 1.0 mol % WO2(acac)2, 1.2 mol % BHA-5 in 0.5 mL DCM.
0.25 equiv NaCl was used.
The reaction was performed on a scale of 10.0 mmol 1a.
