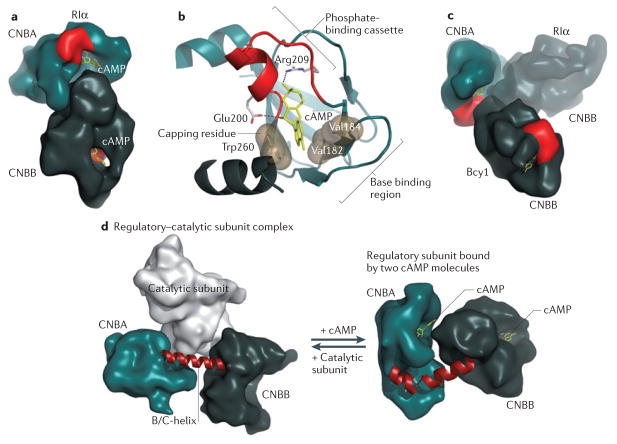
Figure 3. Molecular basis for regulation of PKA by cAMP.
The cyclic nucleotide-binding domains (CNB domains) of cyclic AMP-dependent protein kinase (PKA), which are conserved throughout all species, are defined by a unique fold. a | The two CNB domains (CNBA and CNBB) in RIα in the cAMP-bound state create an extensive network of allosteric communication. b | The conserved features of each CNB domain are summarized. The phosphate-binding cassette (PBC), shown in red, is the signature motif of the CNB domain. The adenine ring of bound cAMP is capped by a hydrophobic residue from within the CNB domain or from associated domains or motifs29. The base-binding region stabilizes the adenine ring by binding on the other side of cAMP. c | Although each CNB domain is highly conserved, the two domains of RIα and Bcy1 (the yeast homologue of RIα) are oriented in distinctly different ways. The overlay of the two structures is shown. d | The remarkable malleability of the regulatory subunit was first recognized when the structure of a regulatory–catalytic subunit complex was solved. On the left is the conformation of the CNB domains (amino acids 91–379 of the regulatory subunit) in the holoenzyme complex, and on the right are the CNB domains bound to two molecules of cAMP. The conformational change is mediated by the B/C-helix in CNBA, which is kinked in the cAMP-bound state and extended into a single long helix in the holoenzyme.