Scheme 2. Model for the Influence of Fluorine Recognition on the Course of the FlK-Catalyzed Reaction.
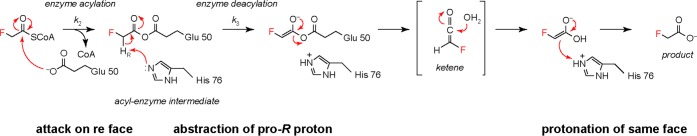
On the basis of the kinetic analysis of substrates with a stereogenic center at the α-carbon, we propose that Glu 50 attacks the fluoroacetyl-CoA thioester bond from the re face. The pro-R proton of the resultant intermediate is then removed by His 76. Following hydration of the putative intermediate, the carboxylic acid enolate is reprotonated on the same face from which the proton was removed.
