Figure 1.
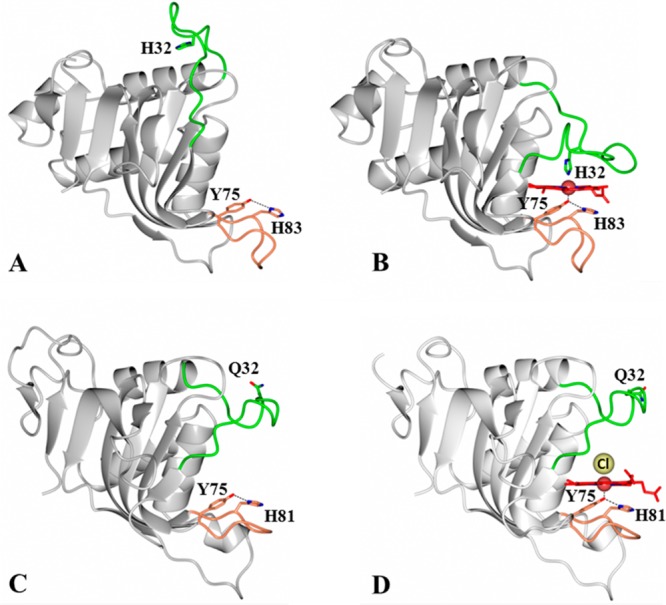
HasA hemophores display structural conservation of the proximal loop (coral) but divergence in the structure and function of the distal loop (green). Structures of (A) apo-HasAp [Protein Data Bank (PDB) entry 3MOK] and (B) holo-HasAp (PDB entry 3ELL) illustrate the large conformational rearrangement of the distal loop (green) caused by heme binding, which results in heme axial ligation by His32 and Tyr75. In contrast, the structures of (C) apo-HasAyp (PDB entry 4JER) and (D) holo-HasAyp (PDB entry 4JET) illustrate the minimal reorganization of the distal loop upon heme binding and the coordination of heme by only one protein-provided ligand, Tyr75. The Cl– ion that coordinates to the distal site is shown as a yellow sphere.
