Figure 11.
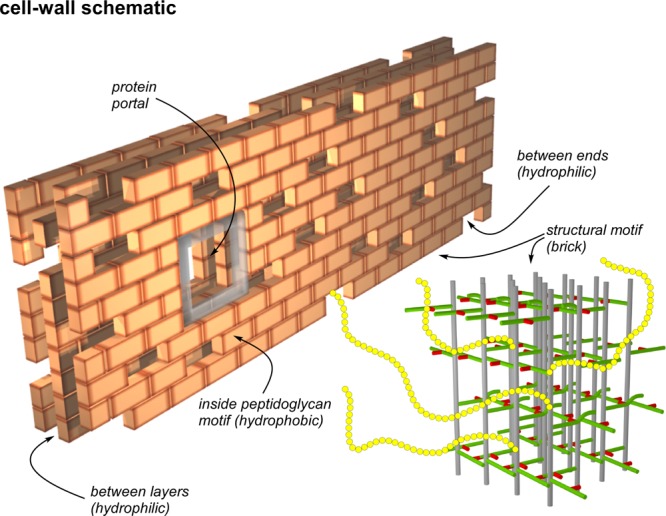
Schematic representation of the cell wall of the FemA mutant of S. aureus as a multilayered brick wall. Each brick is the peptidoglycan structural motif (bottom right) shown in expanded view in Figure 10. The interior of the structural motif is hydrophobic and the potential binding site of glycopeptide drugs with hydrophobic tails. Spaces and gaps are hydrophilic and accommodate wall teichoic acid (yellow chains). This arrangement brings the 31P of phosphate groups close to the surfaces of bricks, which are necessarily rich in un-cross-linked d-alanyl carboxyl groups, consistent with the results of Figure 3 (right). Bricks are placed around membrane-bound proteins creating portals to the cell surface. The membrane bilayer is envisioned parallel to the back surface of the wall which is built one layer at a time with the glycan chains of the structural motif parallel to the bilayer surface, consistent with the results of Figures 6–8.
