Abstract
Identification of enzyme-bound intermediates via their spectroscopic signatures, which then allows direct monitoring of the kinetic fate of these intermediates, poses a continuing challenge. As an electrophilic covalent catalyst, the thiamin diphosphate (ThDP) coenzyme forms a number of noncovalent and covalent intermediates along its reaction pathways, and multiple UV–vis and circular dichroism (CD) bands have been identified at Rutgers pertinent to several among them. These electronic transitions fall into two classes: those for which the conjugated system provides a reasonable guide to the observed λmax and others in which there is no corresponding conjugated system and the observed CD bands are best ascribed to charge transfer (CT) transitions. Herein is reported the reaction of four ThDP enzymes with alternate substrates: (a) acetyl pyruvate, its methyl ester, and fluoropyruvate, these providing the shortest side chains attached at the thiazolium C2 atom and leading to CT bands with λmax values of >390 nm, not pertinent to any on-pathway conjugated systems (estimated λmax values of <330 nm), and (b) (E)-4-(4-chlorophenyl)-2-oxo-3-butenoic acid displaying both a conjugated enamine (430 nm) and a CT transition (480 nm). We suggest that the CT transitions result from an interaction of the π bond on the ThDP C2 side chain as a donor, and the positively charged thiazolium ring as an acceptor, and correspond to covalent ThDP-bound intermediates. Time resolution of these bands allows the rate constants for individual steps to be determined. These CD methods can be applied to the entire ThDP superfamily of enzymes and should find applications with other enzymes.
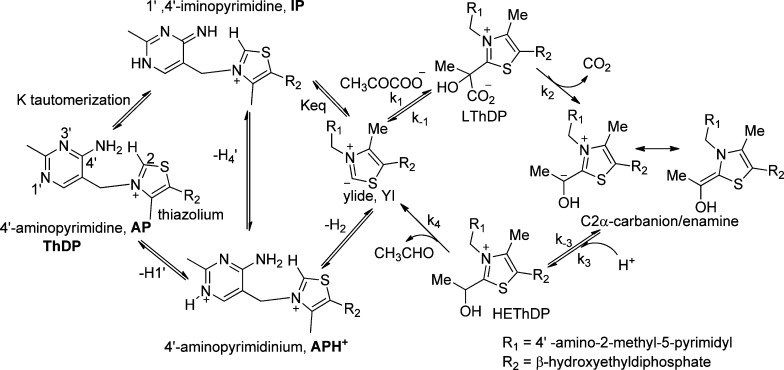
Thiamin diphosphate (ThDP, the vitamin B1 coenzyme) is a cofactor used for biological decarboxylations of 2-oxo acids, as well as C–C bond formations resembling a benzoin condensation, and comprises three distinct chemical moieties, two heteroaromatic rings and a diphosphate group. Early X-ray structural studies of three ThDP enzymes showed that the diphosphate group is important for binding of the cofactor to the enzymes.1−3 Seminal studies of Breslow4 revealed that the thiazolium ring participates as an electrophilic covalent catalyst by conversion of the weak C2-H acid to its conjugate base (called variously a C2 carbanion, a carbene, or an ylide) to initiate the reaction. Only relatively recently was it demonstrated that the 4′-aminopyrimidine ring likely participates in an intramolecular acid–base reaction, probably by assisting the deprotonation at C2-H to generate the nucleophile.5,6 This acid–base function of the 4′-aminopyrimidine is deemed very important in view of the paucity of acid–base catalysts at the active centers of several ThDP enzymes, especially (a) glyoxylate carboligase that lacks even the highly conserved Glu within the short hydrogen bond of the ThDP N1′ atom, or any other potential acid–base catalyst,7 and (b) benzaldehyde lyase, still bearing the conserved Glu side chain, but with only a single His residue in the active center with potential acid–base catalytic function, but too distant from the C2 atom to be directly involved in any proton transfers.8
Typical ThDP enzymes commence the reaction sequence with formation of a covalent nucleophilic adduct of the C2 atom of the thiazolium ring of ThDP with the carbonyl group of the substrate. The decarboxylation of pyruvate to acetaldehyde (Scheme 1) conducted by yeast pyruvate decarboxylase9 (YPDC, EC 4.1.1.1) is shown as an example in Scheme 1. Such reactions proceed by a series of ThDP-bound covalent complexes, including the C2α-tetrahedral predecarboxylation intermediate C2α-lactylThDP (LThDP); the enamine, a C2α-trigonal (first) postdecarboxylation intermediate; and the C2α-tetrahedral (second) postdecarboxylation intermediate C2α-hydroxyethylThDP (HEThDP). Given the chirality of the protein matrix, all enzyme-bound complexes of ThDP, covalent and noncovalent, are chiral and, in theory, can be detected by circular dichroism (CD) spectroscopy. More than four decades ago, the group of Kochetov pioneered application of CD to ThDP enzymes with experiments on transketolase,10,11 in which in the absence of substrate a negative CD band with a maximum near 320 nm was observed. During the past dozen years, the Rutgers group has uncovered multiple spectral signatures for intermediates along the ThDP-dependent enzymatic pathways. These spectral signatures are best seen by CD, where proximal bands are often fortuitously resolvable because they have opposite phases (a property absent in absorption spectra). So far, the following assignments have been made on more than 10 ThDP enzymes: (1) a positive CD band centered near 300–315 nm pertaining to the 1′,4′-iminopyrimidineThDP tautomer (IP form) in either enzyme-bound ThDP12 or intermediates related to the C2α-tetrahedral adducts LThDP and HEThDP at pH values near or above the pKa of the 4′-aminopyrimidinium (APH+) conjugate acid;6,13 (2) a negative CD band centered at 320 nm pertaining to the canonical 4′-aminopyrimidine form (AP form),6,13,14 the band observed by Kochetov and co-workers;10 (3) electronic transitions corresponding to the enamine, which until the discovery of the IP form was the only obvious conjugated ThDP-bound intermediate, whose λmax depends on the group attached to the C2α atom (ranging from 295 nm for CH39,15,16 to ∼380 nm for a phenyl ring17−20 to 430–440 nm for a styryl substituent);21,22 and (4) the Michaelis complex reported by a negative CD band centered around 330–340 nm.12,23 The λmax of the IP form in case (1) and the enamine in case (3) observed on enzymes could be well reproduced in chemical models.5,24 However, those of AP form (2) and Michaelis complex (4) could not, and the band pertinent to the AP form almost certainly originates from a charge transfer (CT) transition between the 4′-aminopyrimidine as a donor and the thiazolium ring as an acceptor.6,13
Scheme 1. Mechanism of Yeast Pyruvate Decarboxylase.
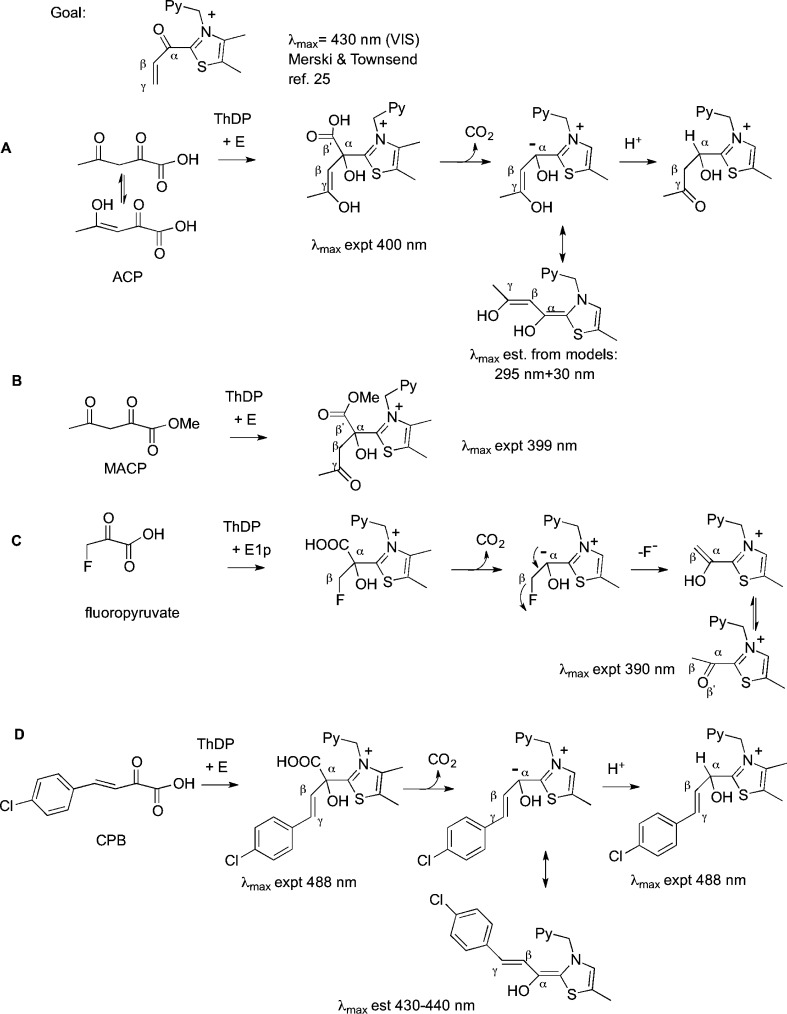
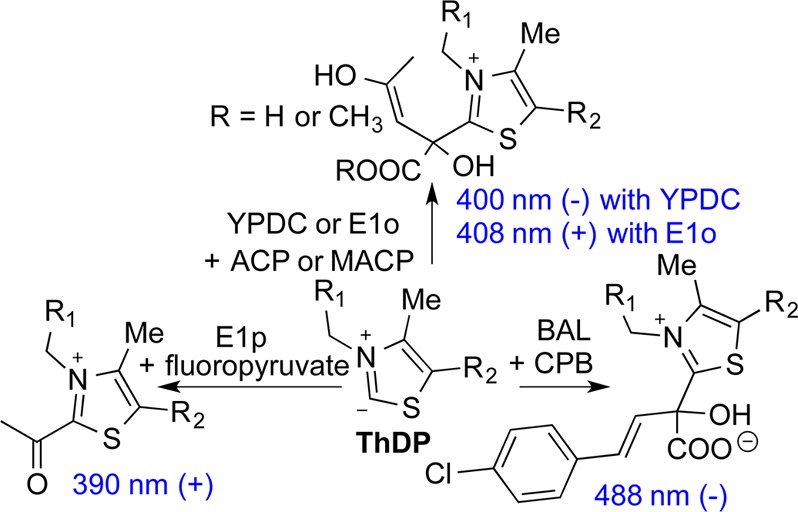
Additionally, we had reported CD spectroscopic detection of ThDP-bound intermediates derived from a chromophoric substrate, (E)-2-oxo-4-(pyridin-3-yl)-3-butenoic acid, on benzaldehyde lyase at wavelengths much longer than expected on the basis of the length of the conjugated system. In view of the information that has been gathered at Rutgers, we were intrigued by the report of Merski and Townsend25 of a vis intermediate with a λmax of 430 nm on the first enzyme in clavulanic acid biosynthesis. This was attributed to 2-acryloylthiamin diphosphate, but that λmax could not be achieved by any of the conjugated synthetic models created.25 Having available a battery of ThDP enzymes, we undertook a study of four alternate 2-oxo acid substrates, acetyl pyruvate (ACP), its methyl ester (MACP), fluoropyruvate, and (E)-4-(4-chlorophenyl)-2-oxo-3-butenoic acid (CPB) (Figure 1). We did this in a search for CT bands that could serve as reporters of ThDP-bound covalent intermediates on enzymes, especially signatures for such intermediates not previously characterized.
Figure 1.
Suggested origins of CT bands formed on ThDP enzymes using (A) ACP, (B) MACP, (C) fluoropyruvate, and (D) CPB.
In addition to YPDC, we used the E1 components of the Escherichia coli pyruvate (E1p) and 2-oxoglutarate (E1o) dehydrogenase complexes, and benzaldehyde lyase (BAL), an enzyme conducting a retro-benzoin condensation from (R)-benzoin to two benzaldehyde molecules (Scheme S1 of the Supporting Information). The four compounds listed were used to elucidate the origin of the CD or photo diode array bands observed at wavelengths longer than expected on the basis of the length of the conjugated system. The compound MACP could form only an LThDP-like predecarboxylation complex, a model for the pre- and postdecarboxylation C2α-tetrahedral intermediates produced by ACP. Upon decarboxylation of fluoropyruvate to the enamine, subsequent fluoride ion elimination is a source of 2-acetylThDP.26,27 Compounds ACP, MACP, and fluoropyruvate create ThDP-bound intermediates with very short conjugations in the C2 side chain, assuring us that all observations above 320–330 nm reflect CT transitions and/or bands, while CPB gives rise to both styryl-type conjugation and CT transitions. The results provide new spectroscopic signatures for ThDP intermediates, including the very important 2-acetylThDP (in general 2-acylThDP), a likely intermediate on the superfamily of 2-oxo acid dehydrogenase multienzyme complexes (Scheme S2 of the Supporting Information).
Experimental Procedures
Materials
Methyl 4-hydroxy-2-oxopent-3-enoate (MACP) and morpholinoethanesulfonic acid (MES) were purchased from Sigma-Aldrich (St. Louis, MO). Hydrolysis of MACP to ACP was achieved by following the procedure mentioned in the Supporting Information. The potassium salt of (E)-4-(4-chlorophenyl)-2-oxo-3-butenoic acid (CPB) was synthesized as previously reported.21 ThDP was purchased from USB (Cleveland, OH).
Circular Dichroism Experiments
All steady state CD spectra were recorded on a Chirascan CD spectrometer from Applied Photophysics (Leatherhead, U.K.) in 2.4 mL volume with a 1 cm path length cell.
pH Titration of E477Q YPDC with Pyruvate Present
E477Q (33.3 μM active centers) in a triple-pH buffer system containing 50 mM acetic acid, 50 mM MES, 100 mM Tris, 0.5 mM ThDP, 5 mM MgCl2, and 10 mM pyruvate was titrated in the pH range of 5.3–6.2 at 5 °C.
Titration of E477Q YPDC with ACP
E477Q YPDC (83.3 μM active centers) was titrated with ACP (0.05–10 mM) in 50 mM MES (pH 6.0) containing 0.5 mM ThDP and 2 mM MgCl2 at 5 °C.
Titration of E477Q YPDC with MACP
E477Q YPDC (33.3 μM active centers) was titrated with MACP (0.05–10 mM) in 50 mM MES (pH 6.0) containing 0.5 mM ThDP and 2 mM MgCl2 at 5 °C.
Titration of E1o with ACP
E1o (33.3 μM active centers) was titrated with ACP (0.05–2 mM) in 20 mM KH2PO4 (pH 7.0) containing 0.2 mM ThDP and 2 mM MgCl2 at 5 °C.
Reaction of E1p and Its H407A and E571A Variants with Fluoropyruvate
E1p (3.0 mg/mL, 30 μM active centers) was reacted with 5 mM fluoropyruvate in 50 mM KH2PO4 (pH 7.0) containing 0.2 mM ThDP and 1 mM MgCl2, and the CD spectra were recorded after different periods of incubation at 25 °C. Fluoropyruvate (15 mM) for the H407A E1p variant (2.5 mg/mL, 25 μM active centers) and fluoropyruvate (10 mM) for the E571A E1p variant (3.0 mg/mL, 30 μM active centers) were used under conditions similar to those used for wild-type E1p.
Titration of YPDC with CPB
YPDC (33.3 μM active centers) was titrated with CPB (0.03–4 mM) in 50 mM MES (pH 6.0) containing 0.5 mM ThDP and 2 mM MgCl2 at 5 °C.
Titration of BAL with CPB
BAL (25.5 μM active centers) was titrated with CPB (0.08–10 mM) in 50 mM Tris (pH 8.0) containing 0.2 mM ThDP and 1 mM MgCl2 at 30 °C. The Kd value was calculated by fitting the data to a Hill function (eq 1).
| 1 |
where CDλ is the observed CD signal at a particular wavelength, CDmaxλ is the maximal CD signal at saturation with ligand, [ligand] is the concentration of substrate, and nH is the Hill coefficient.
Rapid-Scan Stopped-Flow Photodiode Array (PDA) Experiments of BAL with CPB
These experiments were conducted on an SX.18MV stopped-flow spectrophotometer from Applied Photophysics. Experiments were performed by mixing equal volumes of BAL (34.0 μM) and CPB (10 mM). A slit width of 2 mm and a path length of 2 mm were used. Sigma plot 10.0 was used to fit the data using a single-exponential model as in eq 2
| 2 |
| 3 |
where k1 and k2 are the apparent rate constants and c is the Amaxλ in the exponential rise to maximum or decay model.
Results
Formation of the 1′,4′-Iminopyrimidinyl Tautomeric Form of ThDP on YPDC
Previously, we had shown that titrating the slow E477Q YPDC variant with increasing concentrations of pyruvate forms LThDP, the predecarboxylation intermediate, present in its 1′,4′-iminopyrimidylLThDP form (positive CD band at ∼300 nm) and as a Michaelis complex (negative band at 330 nm).28 Increasing the pH from 5.3 to 6.2 and recording the positive CD band at 305 nm formed from pyruvate with E477Q YPDC (1′,4′-iminoLThDP) resulted in an increase in amplitude (Figure S1 of the Supporting Information), suggesting formation of the IP form from the N1-protonated 4′-aminopyridinium (APH+), and implying a pKa near 6.1 for the APH+ form.29
Circular Dichroism Band Formation with ACP and MACP
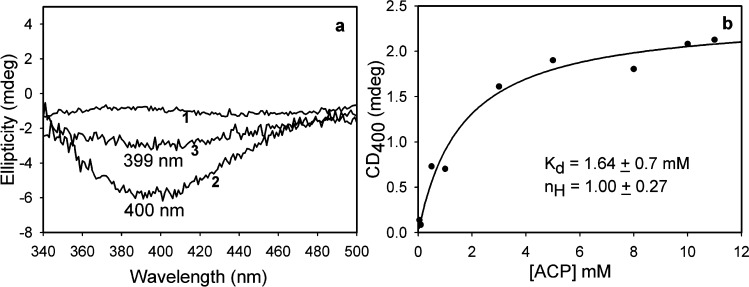
In contrast to the result with pyruvate, CD titration of E477Q YPDC with ACP did not provide evidence of formation of either the IP form or the Michaelis complex; instead, it revealed formation of a negative band at 400 nm that exhibited saturation, a band that increased in amplitude after overnight incubation at 4 °C, thus suggesting a slow reaction. The CD spectrum of the protein after separation of the supernatant confirmed that the species represented by the 400 nm CD band is protein-bound. The negative CD band at 400 nm was assigned to a CT transition based on the following. The 400 nm band could not correspond to the enamine because the enamine derived from pyruvic acid has a λmax of 295 nm,15 and even an additional double bond could not shift the λmax beyond 330 nm. The CT band (Figure 1A) is believed to correspond to the predecarboxylation intermediate on ThDP with a Kd of 1.64 mM (Figure 2). A similar experiment conducted with E1o also revealed the formation of a CD band at 408 nm, but in this case with a positive phase (Figure S2 of the Supporting Information). Next, a CD titration of E477Q YPDC was also conducted at 5 °C with MACP, the methyl ester of acetopyruvate, which after overnight incubation also formed a negative CD band at 399 nm (Figure 2), at the same wavelength as with ACP. The weak signal at 399 nm was confirmed to be protein-bound, as it persisted in a spectrum after the protein had been separated from supernatant and redissolved in fresh buffer.
Figure 2.
(a) Formation of a CD charge transfer band on YPDC at 5 °C: (1) E477Q YPDC enzyme, (2) E477Q with ACP, and (3) E477Q YPDC with MACP. (b) Data from the experiment with E477Q YPDC and ACP were fit to a Hill function [eq 1 (see Experimental Procedures)].
Formation of the intermediate appears to be very slow with MACP (ester), while ACP undergoes slow turnover forming a steady state level of intermediate saturating the available active centers. These results suggest that the similar CD band observed with both ACP and MACP (Figure 2a) pertains to the same predecarboxylation intermediate because the ThDP–MACP adduct could not be decarboxylated (Figure 1B). Irrespective of whether the CD band with ACP pertains to the pre- or postdecarboxylation C2α-tetrahedral intermediate (these could be differentiated according to their C6′-H 1H chemical shifts30), its wavelength excludes the enamine and clearly demonstrates the possibility of forming ThDP intermediates with λmax at 400 nm in the absence of significant conjugation. This band likely corresponds to a CT transition in origin.
Reaction of Fluoropyruvate with E1p Leads to the Formation of a New CD Band Corresponding to 2-AcetylThDP
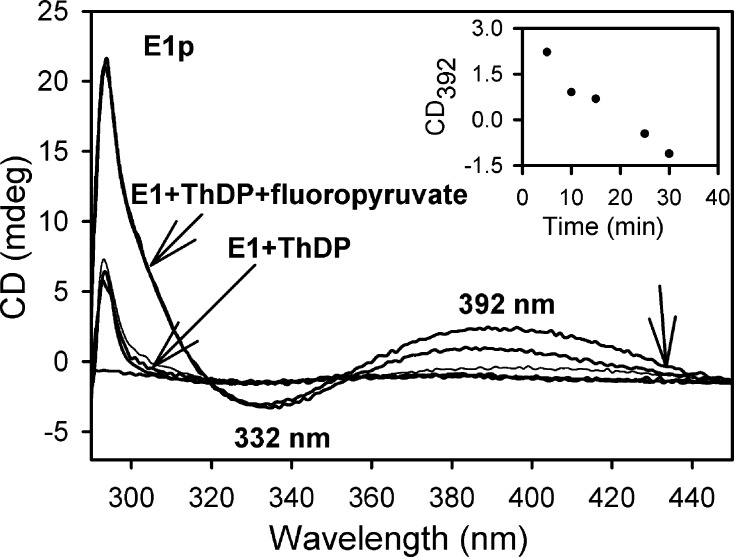
It had been reported by Frey and co-workers that fluoropyruvate is a convenient source of 2- acetylThDP on E1p (Figure 1C). Following decarboxylation to the enamine, fluoride ion elimination leads to the enol form of 2-acetylThDP, which then tautomerizes to the keto form.26,27 CD spectra of E1p and some of its low-activity active center variants revealed formation of a very broad new positive band near 390–395 nm (Figure 3), which was assigned to the enzyme-bound 2-acetylThDP. It has been amply demonstrated in the literature that, in model reactions, this intermediate can undergo rapid hydrolysis to acetate ion. Hence, its short lifetime is not surprising, and in fact, the rate of decomposition appears to be similar in E1p and in its slow H407A and E571A variants (Figure S3 of the Supporting Information). This acetyl group is the shortest side chain at the ThDP C2 position in our list in Table 1, yet it still has a long λmax (390 nm) CT transition. This is our closest model for the Merski–Townsend observation attributed to 2-acryloylThDP. It is not unreasonable that replacement of the methyl group with a vinyl group, an extension of the π system by a C=C double bond, according to the Woodward–Fieser rules,31,32 would account for the observed λmax of 430 nm (390 nm + 30 nm = 420 nm).
Figure 3.
Circular dichroism spectra of E1p in the presence of 5 mM fluoropyruvate. The inset shows the time dependence of the CD band at 392 nm.
Table 1. CT Bands Related to ThDP Observed by CD Spectroscopy in This Study.
| enzyme | substrate | CT band (nm) (phase) | assignments |
|---|---|---|---|
| E477Q YPDC | ACP | 400 (−) | predecarboxylation |
| E1o | ACP | 408 (+) | predecarboxylation |
| E477Q YPDC | MACP | 399 (−) | predecarboxylation |
| E1p | fluoropyruvate | 390 (+) | 2-acetyllThDP |
| BAL | CPB | 488 (−), 434 (+) | predecarboxylation and enamine |
Formation of a Long Wavelength CT Band with a Longer Conjugated Substrate, CPB
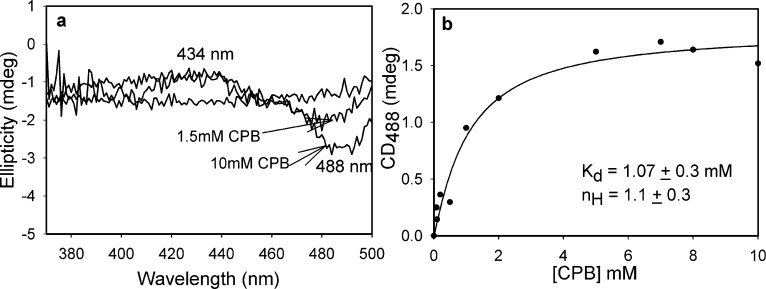
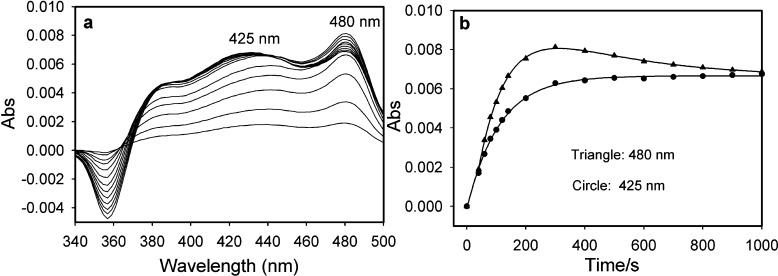
CPB was the first conjugated 2-oxo acid giving evidence of a YPDC-bound enamine intermediate (λmax at 440 nm)21 and is also a suicide substrate with YPDC.33 Here we used CD to observe the formation of the enamine on YPDC from CPB as a negative band at 440 nm (Figure S4 of the Supporting Information). Thus, both vis spectroscopy and CD suggest that the enamine derived from ThDP-CPB on YPDC has a λmax of 440 nm, while the CD experiment also confirms that the ThDP-bound enamine is chiral, as expected. Unlike that with YPDC, reaction of CPB with BAL at 30 °C formed a negative CT band at 488 nm and a positive one at 434 nm [Kd = 1.1 mM (Figure 4)]. These results are similar to those obtained by our groups with (E)-2-oxo-4-(pyridin-3-yl)-3-butenoic acid. In that instance, the band near 480 nm was assigned to the LThDP-type predecarboxylation intermediate (Figure 1D).20 A stopped-flow UV–vis photodiode array experiment was conducted by mixing BAL (34 μM active centers) in one syringe with an equal volume of 10 mM CPB placed in the second syringe at 30 °C. As with CD, time-dependent changes were observed at 480 and 430 nm (Figure 5). This provided the following rate constants: k1 = 0.0098 ± 0.0007 s–1 (formation of the LThDP-like intermediate), k2 = 0.0031 ± 0.0007 s–1 (formation of the HEThDP-like intermediate from the enamine), and k1 = 0.0090 ± 0.0002 s–1 (formation of the enamine) (Figure 5). The rates indicate that conversion of the LThDP-like intermediate (488 nm) to the enamine (434 nm) is very fast compared to depletion of the enamine to the HEThDP-like intermediate. Both the CD and stopped-flow PDA analysis confirmed that CPB on BAL forms a tetrahedral covalent adduct with ThDP, leading to the appearance of a CT band. An X-ray structure of (E)-2-oxo-4-(pyridin-3-yl)-3-butenoic acid cocrystallized with benzoylformate decarboxylase revealed the stability of the postdecarboxylation HEThDP-like intermediate [the C2 adduct of ThDP and (E)-3-(pyridin-3-yl)acrylaldehyde], also confirming fast decarboxylation.20 Quantitative analysis of the kinetic data in Figure 5 is made difficult because the LThDP- and HEThDP-like C2α-tetrahedral intermediates derived from (E)-2-oxo-4-(pyridin-3-yl)-3-butenoic acid have very similar λmax values (477 and 473 nm in vis photodiode array spectra, respectively).20
Figure 4.
(a) Formation of the CT band by CD titration of BAL with CPB at 30 °C. (b) Data were fit to a Hill function [eq 1 (see Experimental Procedures)].
Figure 5.
(a) Time-dependent formation of a CT band on BAL with CPB detected by a stopped-flow photodiode array at 30 °C. (b) Data were fit using eq 2 (425 nm) and eq 3 (480 nm) (see Experimental Procedures for reaction conditions and equations).
Discussion
In this study, CD spectroscopy was used to identify the CT signal corresponding to intermediates derived from substrates covalently bound to ThDP on four different enzymes. A summary of the steady state formation of ThDP-related intermediates reflected by formation of CT bands is given in Table 1 and Figure 1.
It was shown that the predecarboxylation intermediate upon addition of pyruvate to E477Q YPDC is preferentially in its IP tautomeric form and the pKa for the APH+ form is 6.1, very near the pH of optimal activity of 6.2.34 This finding provides the eighth example of the suggestion that at the optimal pH for activity, all ionization states and tautomeric forms of ThDP (APH+, IP, and AP) may be needed in the mechanism,29 ensuring that all are available for catalysis.
With the examples revealed here, we have now identified CD spectroscopic signatures for nearly all covalent and noncovalent ThDP-related intermediates in Scheme 1 and Figure 1. The substrates selected for this study helped us to assign signatures to intermediates at wavelengths longer than 380 nm, well beyond the spectrum of the protein. For instance, ACP and its methyl ester MACP with E477Q formed a similar negative CD band near ∼400 nm. Because MACP could not undergo decarboxylation, the signal must correspond to a predecarboxylation intermediate. The λmax of ∼400 nm could not correspond to a conjugated π system (only adding an acetyl group to pyruvate), strongly implying some other system, such as a CT interaction. Similarly, the very broad CD band at 390 nm formed from fluoropyruvate with E1p corresponds to 2-acetylThDP, a signature never before observed by CD spectroscopy, which also likely represents a CT transition. With this result, the Merski–Townsend observation25 of an intermediate with a λmax near 430 nm on the first enzyme in clavulanate biosynthesis can be assigned with confidence to 2-acryloylThDP and attributed to a CT transition originating from an interaction of the positively charged thiazolium ring with the π bond of the ThDP C2 substituent. From the limited number of examples available to us, we could speculate that the Woodward–Fieser rules also apply to the shift in the λmax of the CT bands. For example, addition of the styryl substituent of CPB to the MACP in the conjugated system (or indeed to the acetyl in 2-acetylThDP) leads to a shift from 390 to 400 to 488 nm.
Attribution of some of the CD bands to charge transfer transitions is also supported by nonenzymatic models using UV–vis spectroscopy. The negative CD band at 320 nm that we attribute to the AP form was assigned to a CT interaction of the 4′-aminopyrimidine ring as an electron donor with the thiazolium ring as an acceptor. This was accomplished by surveying many E1p variants, which indicated that there is no need for an aromatic side chain in the vicinity of ThDP for us to observe the AP form, in contrast to what had been previously believed. For reasons only theory can address, the CD signals for the CT bands are very much stronger on the enzymes than in models. In a paper by Nemeria et al.,13 we searched for CT bands generated by addition of a thiazolium salt to 4-aminopyrimidine. We could indeed observe such an absorbance with a λmax of 340 nm, but 200 mM thiazolium triflate and at least 30 mM 4-aminopyrimidine were required to produce as much as 30 mA. On ThDP enzymes, we could conduct entire pH titrations of the AP form by CD at an enzyme concentration of 30 μM. This model experiment does provide support for assigning the CD band for the AP form to a CT transition. We have also observed that the CD bands we attribute to CT transitions tend to be much broader than those originating from extended conjugation.
In conclusion, there are now characterized CT bands for both pre- and postdecarboxylation C2α-tetrahedral ThDP-bound intermediates, which can also be differentiated using the NMR method invented by Tittmann and Hübner.30 The remaining ThDP-related species needing spectral signatures are the C2-carbanion/ylide/carbene and the APH+ form. The presence of the APH+ form was established by solid state NMR on three ThDP enzymes.35 Recently, Tittmann’s group reported X-ray evidence suggestive of the existence of the carbene on pyruvate oxidase.36 With our CD assignments in hand, stopped-flow CD can now be exploited to provide rate constants for individual steps on the reaction pathways. This has been demonstrated recently on three ThDP-dependent enzymes, E1p,37 glyoxylate carboligase,38 and 1-deoxy-d-xylulose-5-phosphate synthase.39 It is also worth emphasizing that with the ready availability of high-sensitivity steady state and stopped-flow CD instrumentation, the methods here used could indeed be applied to other enzymes, as such signatures for intermediates are so much more readily quantified by CD than by UV–vis spectroscopy.
Glossary
Abbreviations
- ThDP
thiamin diphosphate
- YPDC
yeast pyruvate decarboxylase
- E1o
E1 component of the E. coli 2-oxoglutarate dehydrogenase complex
- E1p
E1 component of the E. coli pyruvate dehydrogenase complex
- BAL
benzaldehyde lyase
- ACP
acetyl pyruvate
- MACP
methyl acetyl pyruvate
- CPB
(E)-4-(4-chlorophenyl)-2-oxo-3-butenoic acid
- 3-PKB
(E)-2-oxo-4-(pyridin-3-yl)-3-butenoic acid
- CT
charge transfer transition, a result of an electronic transition to an excited state in which there is a partial transfer of electronic charge from the donor to the acceptor moiety
- CD
circular dichroism
- PDA
photodiode array
- Yl
C2-carbanion/ylide/carbene (known by all three names) conjugate base form of ThDP
- IP
1′,4′-iminopyrimidine tautomeric form of ThDP
- AP
4′-aminopyrimidine tautomeric form of ThDP
- APH+
4′-aminopyrimidinium conjugate acid form of ThDP
- LThDP
C2α-lactyl ThDP
- NADH
reduced nicotinamide adenine dinucleotide
- YADH
yeast alcohol dehydrogenase
- DCPIP
2,6-dichlorophenolindophenol
- MES
2-(N-morpholino)ethanesulfonic acid.
Supporting Information Available
Synthesis of acetyl pyruvate, enzyme purification, activity assays, two schemes, four figures, and references. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
This work was supported by National Institutes of Health Grant GM050380 (F.J.) and Indiana University-Purdue University Indianapolis startup funds (M.J.M.).
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Muller Y. A.; Schulz G. E. (1993) Structure of the thiamine- and flavin-dependent enzyme pyruvate oxidase. Science 259, 965–967. [DOI] [PubMed] [Google Scholar]
- Lindqvist Y.; Schneider G.; Ermler U.; Sundstrom M. (1992) Three-dimensional structure of transketolase, a thiamine diphosphate dependent enzyme, at 2.5 Å resolution. EMBO J. 11, 2373–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyda F.; Furey W.; Swaminathan S.; Sax M.; Farrenkopf B.; Jordan F. (1993) Catalytic centers in the thiamin diphosphate dependent enzyme pyruvate decarboxylase at 2.4-Å resolution. Biochemistry 32, 6165–6170. [DOI] [PubMed] [Google Scholar]
- Breslow R. (1957) Rapid deuterium exchange in thiazolium salts. J. Am. Chem. Soc. 79, 1762–1763. [Google Scholar]
- Jordan F.; Zhang Z.; Sergienko E. (2002) Spectroscopic evidence for participation of the 1′,4′-imino tautomer of thiamin diphosphate in catalysis by yeast pyruvate decarboxylase. Bioorg. Chem. 30, 188–198. [DOI] [PubMed] [Google Scholar]
- Jordan F.; Nemeria N. S.; Zhang S.; Yan Y.; Arjunan P.; Furey W. (2003) Dual catalytic apparatus of the thiamin diphosphate coenzyme: Acid-base via the 1′,4′-iminopyrimidine tautomer along with its electrophilic role. J. Am. Chem. Soc. 125, 12732–12738. [DOI] [PubMed] [Google Scholar]
- Kaplun A.; Binshtein E.; Vyazmensky M.; Steinmetz A.; Barak Z.; Chipman D. M.; Tittmann K.; Shaanan B. (2008) Glyoxylate carboligase lacks the canonical active site glutamate of thiamine-dependent enzymes. Nat. Chem. Biol. 4, 113–118. [DOI] [PubMed] [Google Scholar]
- Mosbacher T. G.; Mueller M.; Schulz G. E. (2005) Structure and mechanism of the ThDP-dependent benzaldehyde lyase from Pseudomonas fluorescens. FEBS J. 272, 6067–6076. [DOI] [PubMed] [Google Scholar]
- Jordan F. (2003) Current mechanistic understanding of thiamin diphosphate-dependent enzymatic reactions. Nat. Prod. Rep. 20, 184–201. [DOI] [PubMed] [Google Scholar]
- Kochetov G. A.; Usmanov R. A.; Merzlov V. P. (1970) Thiaminepyrophosphate induced changes in the optical activity of baker’s yeast transketolase. FEBS Lett. 9, 265–266. [DOI] [PubMed] [Google Scholar]
- Usmanov R. A. M. L., Neef H., Schellenberger A., and Kochetov G. A. (1996) Interaction of the transketolase with thiamine pyrophosphate analogs. In Biochemistry and Physiology of Thiamin Diphosphate Enzymes (Bisswanger H., and Schellenberger A., Eds.) pp 516–531, A. u. C. Intemann Verlag, Prien, Germany. [Google Scholar]
- Nemeria N.; Chakraborty S.; Baykal A.; Korotchkina L. G.; Patel M. S.; Jordan F. (2007) The 1′,4′-iminopyrimidine tautomer of thiamin diphosphate is poised for catalysis in asymmetric active centers on enzymes. Proc. Natl. Acad. Sci. U.S.A. 104, 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeria N.; Baykal A.; Joseph E.; Zhang S.; Yan Y.; Furey W.; Jordan F. (2004) Tetrahedral intermediates in thiamin diphosphate-dependent decarboxylations exist as a 1′,4′-imino tautomeric form of the coenzyme, unlike the Michaelis complex or the free coenzyme. Biochemistry 43, 6565–6575. [DOI] [PubMed] [Google Scholar]
- Nemeria N. S.; Chakraborty S.; Balakrishnan A.; Jordan F. (2009) Reaction mechanisms of thiamin diphosphate enzymes: Defining states of ionization and tautomerization of the cofactor at individual steps. FEBS J. 276, 2432–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan F.; Kudzin Z. H.; Rios C. B. (1987) Generation and physical-properties of enamines related to the key intermediate in thiamin diphosphate dependent enzymatic pathways. J. Am. Chem. Soc. 109, 4415–4416. [Google Scholar]
- Jordan F.; Nemeria N. S. (2005) Experimental observation of thiamin diphosphate-bound intermediates on enzymes and mechanistic information derived from these observations. Bioorg. Chem. 33, 190–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta G.; Huskey W. P.; Jordan F. (1992) Observation of a 2α-enamine from a 2-(methoxyphenylmethyl)-3,4-dimethylthiazolium salt in water: Implications for catalysis by thiamin diphosphate-dependent α-keto acid decarboxylases. J. Am. Chem. Soc. 114, 7607–7608. [Google Scholar]
- Barletta G. L.; Zou Y.; Huskey W. P.; Jordan F. (1997) Kinetics of C(2α)-proton abstraction from 2-benzylthiazolium salts leading to enamines relevant to catalysis by thiamin-dependent enzymes. J. Am. Chem. Soc. 119, 2356–2362. [Google Scholar]
- Chakraborty S.; Nemeria N.; Yep A.; McLeish M. J.; Kenyon G. L.; Jordan F. (2008) Mechanism of benzaldehyde lyase studied via thiamin diphosphate-bound intermediates and kinetic isotope effects. Biochemistry 47, 3800–3809. [DOI] [PubMed] [Google Scholar]
- Chakraborty S.; Nemeria N. S.; Balakrishnan A.; Brandt G. S.; Kneen M. M.; Yep A.; McLeish M. J.; Kenyon G. L.; Petsko G. A.; Ringe D.; Jordan F. (2009) Detection and time course of formation of major thiamin diphosphate-bound covalent intermediates derived from a chromophoric substrate analogue on benzoylformate decarboxylase. Biochemistry 48, 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo D. J.; Jordan F. (1983) Direct spectroscopic observation of a brewer’s yeast pyruvate decarboxylase-bound enamine intermediate produced from a suicide substrate. Evidence for nonconcerted decarboxylation. J. Biol. Chem. 258, 13415–13417. [PubMed] [Google Scholar]
- Zeng X.; Chung A.; Haran M.; Jordan F. (1991) Direct observation of the kinetic fate of a thiamin diphosphate bound enamine intermediate on brewers-yeast pyruvate decarboxylase: Kinetic and regiospecific consequences of allosteric activation. J. Am. Chem. Soc. 113, 5842–5849. [Google Scholar]
- Kale S.; Ulas G.; Song J.; Brudvig G. W.; Furey W.; Jordan F. (2008) Efficient coupling of catalysis and dynamics in the E1 component of Escherichia coli pyruvate dehydrogenase multienzyme complex. Proc. Natl. Acad. Sci. U.S.A. 105, 1158–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykal A. T.; Kakalis L.; Jordan F. (2006) Electronic and nuclear magnetic resonance spectroscopic features of the 1′,4′-iminopyrimidine tautomeric form of thiamin diphosphate, a novel intermediate on enzymes requiring this coenzyme. Biochemistry 45, 7522–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merski M.; Townsend C. A. (2007) Observation of an acryloyl-thiamin diphosphate adduct in the first step of clavulanic acid biosynthesis. J. Am. Chem. Soc. 129, 15750–15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flournoy D. S.; Frey P. A. (1986) Pyruvate dehydrogenase and 3-fluoropyruvate: Chemical competence of 2-acetylthiamin pyrophosphate as an acetyl group donor to dihydrolipoamide. Biochemistry 25, 6036–6043. [DOI] [PubMed] [Google Scholar]
- Flournoy D. S.; Frey P. A. (1989) Inactivation of the pyruvate dehydrogenase complex of Escherichia coli by fluoropyruvate. Biochemistry 28, 9594–9602. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A.; Gao Y.; Moorjani P.; Nemeria N. S.; Tittmann K.; Jordan F. (2012) Bifunctionality of the thiamin diphosphate cofactor: Assignment of tautomeric/ionization states of the 4′-aminopyrimidine ring when various intermediates occupy the active sites during the catalysis of yeast pyruvate decarboxylase. J. Am. Chem. Soc. 134, 3873–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeria N.; Korotchkina L.; McLeish M. J.; Kenyon G. L.; Patel M. S.; Jordan F. (2007) Elucidation of the chemistry of enzyme-bound thiamin diphosphate prior to substrate binding: Defining internal equilibria among tautomeric and ionization states. Biochemistry 46, 10739–10744. [DOI] [PubMed] [Google Scholar]
- Tittmann K.; Golbik R.; Uhlemann K.; Khailova L.; Schneider G.; Patel M.; Jordan F.; Chipman D. M.; Duggleby R. G.; Hubner G. (2003) NMR analysis of covalent intermediates in thiamin diphosphate enzymes. Biochemistry 42, 7885–7891. [DOI] [PubMed] [Google Scholar]
- Woodward R. B. (1941) Structure and the absorption spectra of α,β-unsaturated ketones. J. Am. Chem. Soc. 63, 1123–1126. [Google Scholar]
- Fieser L. F., and Fieser M. (1949) Natural Products Related to Phenanthrene, Reinhold, New York. [Google Scholar]
- Kuo D. J.; Jordan F. (1983) Active site directed irreversible inactivation of brewers’ yeast pyruvate decarboxylase by the conjugated substrate analogue (E)-4-(4-chlorophenyl)-2-oxo-3-butenoic acid: Development of a suicide substrate. Biochemistry 22, 3735–3740. [DOI] [PubMed] [Google Scholar]
- Jordan F.; Kuo D. J.; Monse E. U. (1978) A pH-rate determination of the activity-pH profile of enzymes. Application to yeast pyruvate decarboxylase demonstrating the existence of multiple ionizable groups. Anal. Biochem. 86, 298–302. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A.; Paramasivam S.; Chakraborty S.; Polenova T.; Jordan F. (2012) Solid-state nuclear magnetic resonance studies delineate the role of the protein in activation of both aromatic rings of thiamin. J. Am. Chem. Soc. 134, 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D.; Neumann P.; Ficner R.; Tittmann K. (2013) Observation of a stable carbene at the active site of a thiamin enzyme. Nat. Chem. Biol. 9, 488–490. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A.; Nemeria N. S.; Chakraborty S.; Kakalis L.; Jordan F. (2012) Determination of pre-steady-state rate constants on the Escherichia coli pyruvate dehydrogenase complex reveals that loop movement controls the rate-limiting step. J. Am. Chem. Soc. 134, 18644–18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeria N.; Binshtein E.; Patel H.; Balakrishnan A.; Vered I.; Shaanan B.; Barak Z.; Chipman D.; Jordan F. (2012) Glyoxylate carboligase: A unique thiamin diphosphate-dependent enzyme that can cycle between the 4′-aminopyrimidinium and 1′,4′-iminopyrimidine tautomeric forms in the absence of the conserved glutamate. Biochemistry 51, 7940–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H.; Nemeria N. S.; Brammer L. A.; Freel Meyers C. L.; Jordan F. (2012) Observation of thiamin-bound intermediates and microscopic rate constants for their interconversion on 1-deoxy-d-xylulose 5-phosphate synthase: 600-fold rate acceleration of pyruvate decarboxylation by d-glyceraldehyde-3-phosphate. J. Am. Chem. Soc. 134, 18374–18379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.