Figure 7.
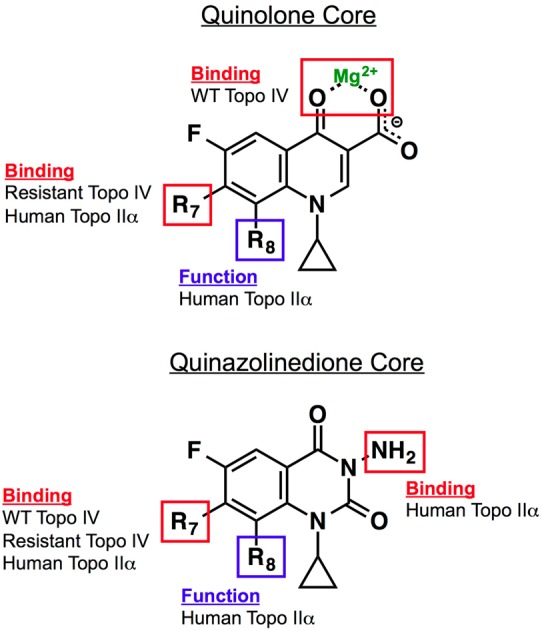
Roles of substituents and core elements of quinolones and quinazolinediones that mediate drug activity against bacterial and human type II topoisomerases. Results are based on studies with B. anthracis topoisomerase IV and human topoisomerase IIα.53 For quinolones, the binding of clinically relevant drugs to topoisomerase IV is mediated primarily through the water–metal ion bridge. The binding of quinolones that overcome resistance is mediated primarily by the substituent at C7. Binding of quinolones to human topoisomerase IIα also is mediated by the C7 substituent. The group at C8 affects the ability of quinolones to act against the human type II enzyme but is not required for drug binding. For quinazolinediones, interactions between drugs and topoisomerase IV (wild-type and resistant) are mediated through the C7 substituent. The effects of the C7 and C8 substituents on quinazolinedione activity against topoisomerase IIα are the same as described for the quinolones. The N3 amino group plays a role in the binding of quinazolinediones to the human enzyme. Adapted from ref (53).
