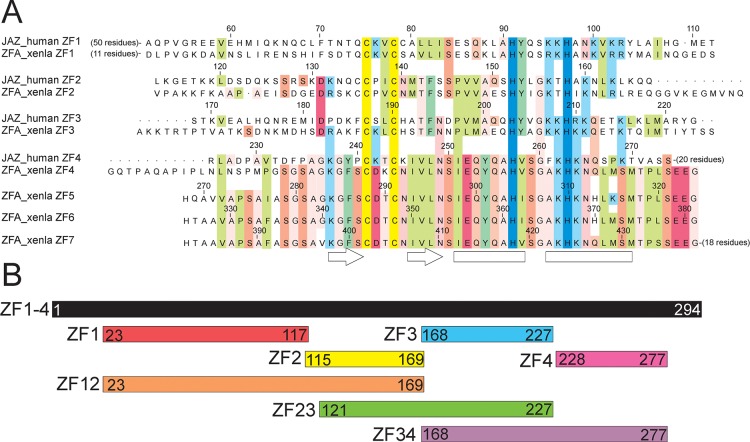
Figure 1.
(A) Partial sequence alignment of the zinc finger domains of human JAZ with each other and with those of Xenopus laevis ZFa. The sequences are aligned on the highly conserved zinc-binding cysteine and histidine residues in each finger, which are colored yellow and blue, respectively. Sequence identity and similarity between fingers are indicated by colored blocks: green for hydrophobic, blue-green for aromatic, light blue for lysine and arginine, red for aspartate and glutamate, and pink for small and hydrophilic residues. Residue numbers in ZF1–4 correspond to the JAZ sequence, and for ZFa, ZF5–7 correspond to the ZFa sequence. Secondary-structure elements present in the zinc finger domains are indicated at the bottom of the figure (arrow, β-strand; box, α-helix). (B) Schematic representation showing the length of the JAZ zinc finger constructs and the N- and C-terminal residue numbers.