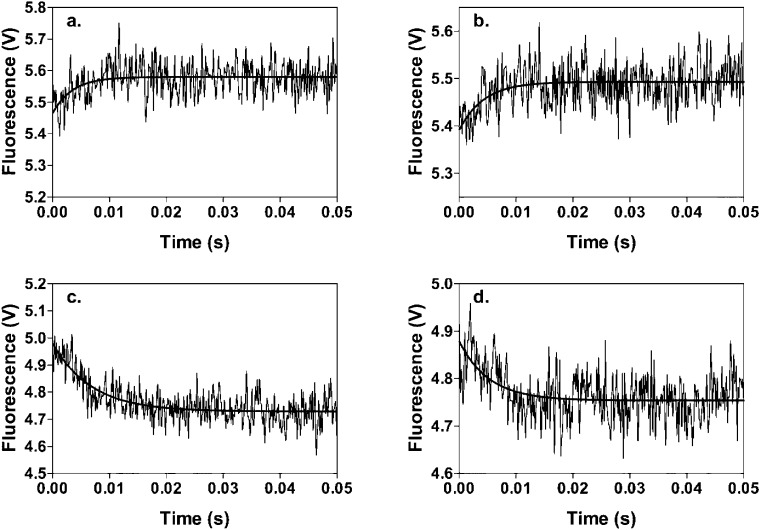
Figure 4.
Time courses of stopped-flow fluorescence experiments to measure either the kinetics of binding of MgFTP to (a) wild-type RePC and (b) mutant H216N or the kinetics of dissociation of (c) the wild-type RPC·MgFTP complex and (d) the H216N·MgFTP complex on displacement by MgATP. Reactions were performed at 30 °C. For panels a and b, 20 μM MgFTP was present in one syringe and 1 μM enzyme in the other. For panels c and d, 20 μM MgFTP and 1 μM wild-type RePC or H216N were present in one syringe and 2 mM MgATP was present in the other. Other reaction conditions were as described in Experimental Procedures.