Summary
Background
Fever with reduced consciousness is an important cause of hospital admission of children in sub-Saharan Africa, with high mortality. Cerebral malaria, diagnosed when acute Plasmodium falciparum infection and coma are recorded with no other apparent reason, is one important cause. We investigated whether viruses could also be an important cause of CNS infection in such patients, and examined the relative contribution of viral pathogens and malaria parasitaemia.
Methods
We did a prospective cohort study in Blantyre, Malawi. From March 1, 2002, to Aug 31, 2004, we enrolled children aged between 2 months and 15 years who were admitted to hospital with suspected non-bacterial CNS infections. Children with a cerebrospinal fluid (CSF) white cell count of less than 1000 cells per μL and negative bacterial microscopy and culture were deemed to have suspected viral CNS infection. Blood was examined for asexual forms of P falciparum. PCR was done on CSF or on post-mortem brain biopsy specimens to detect 15 viruses known to cause CNS infection.
Findings
Full outcome data were available for 513 children with suspected viral CNS infection, of whom 94 (18%) died. 163 children (32%) had P falciparum parasitaemia, of whom 34 (21%) died. At least one virus was detected in the CNS in 133 children (26%), of whom 43 (33%) died. 12 different viruses were detected; adenovirus was the most common, affecting 42 children; mumps, human herpes virus 6, rabies, cytomegalovirus, herpes simplex virus 1, and enterovirus were also important. 45 (9%) of the 513 children had both parasitaemia and viral infection, including 27 (35%) of 78 diagnosed clinically with cerebral malaria. Children with dual infection were more likely to have seizures than were those with parasitaemia alone, viral infection only, or neither (p<0·0001). 17 (38%) of the 45 children with dual infection died, compared with 26 (30%) of 88 with viral infection only, 17 (14%) of 118 with parasitaemia only, and 34 (13%) of 262 with neither (p<0·0001). Logistic regression showed children with a viral CNS infection had a significantly higher mortality than did those who did not have a viral CNS infection (p=0·001).
Interpretation
Viral CNS infections are an important cause of hospital admission and death in children in Malawi, including in children whose coma might be attributed solely to cerebral malaria. Interaction between viral infection and parasitaemia could increase disease severity.
Funding
Wellcome Trust, US National Institutes of Health, and UK Medical Research Council.
Introduction
Febrile illness with reduced consciousness is one of the most important reasons for acute hospital admission of children in sub-Saharan Africa. One of the most common causes is cerebral malaria, which is due to sequestration of parasitised erythrocytes in the cerebral microvasculature. Children are clinically diagnosed with this disorder if asexual forms of Plasmodium falciparum parasites are seen on a peripheral blood film, and if they are in an unrousable coma not attributable to any other cause.1 However, asymptomatic parasitaemia is common, occurring in up to 70% of children in sub-Saharan Africa.2 Therefore, other possible causes of coma—eg, seizures, metabolic derangements, and other infections—must be excluded before coma can be attributed to parasites alone. Previous studies have been focused on superimposed bacterial infections, particularly bacterial meningitis.3 Fundoscopic examination has helped to distinguish children who have retinal changes consistent with autopsy-proven cerebral malaria, from those who do not.4
Little attention has been paid to the possible role of viral CNS infections in comatose children in sub-Saharan Africa.5 We investigated whether viruses could be an important cause of CNS infection, are sometimes the cause of retinopathy-negative cerebral malaria, and could interact with malaria parasites to increase disease severity.
Methods
Study design and participants
We did a prospective cohort study in the paediatric unit of the Queen Elizabeth Hospital in Blantyre, Malawi—an area where P falciparum malaria is endemic. From March 1, 2002, to Aug 31, 2004, we enrolled children aged between 2 months and 15 years who had a suspected CNS infection. CNS infections were suspected in children with a fever or history of fever, who also met at least one of eight additional criteria: reduced level of consciousness (Blantyre coma score6 [BCS] ≤4 for children aged 10 years or younger; or Glasgow coma score [GCS] ≤14 for children older than 10 years); neck stiffness; photophobia; Kernig's sign; tense fontanelle; focal neurological signs; irritability (an inconsolable high pitched cry in a listless child); or convulsions other than simple febrile convulsions.7 We did not include children with simple febrile convulsion because most recover rapidly and this illness is less of a public health burden than are CNS infections.
The protocol was approved by the College of Medicine Research and Ethics Committee, Malawi, and the Ethics Committee of the Liverpool School of Tropical Medicine, UK. The study was explained to accompanying relatives of participating children, who provided written informed consent.
Procedures
On admission to hospital, children with a suspected CNS infection underwent a lumbar puncture. Cerebrospinal fluid (CSF) was taken for biochemical and bacteriological analysis, as described previously,8 and subsequent virological investigations. We excluded children from further study if they had bacterial meningitis proven by Gram stain or bacterial culture, or suspected on the basis of a CSF white cell count of at least 1000 cells per μL or a positive blood culture. We did not include children with suspected bacterial meningitis because they have already been much studied in our setting,9 and the number of additional cases of viral CNS infection was likely to be low.
Children with a CSF white cell count of less than 1000 cells per μL and negative bacterial microscopy and culture were deemed to have suspected viral CNS infection. A member of the study team obtained a detailed history and did a detailed systemic and neurological examination on admission to hospital and at least twice daily until discharge or death. Details were recorded on a standard form. We defined deep coma as a BCS of 2 or less or a GCS of 8 or less, depending on the age of the child.6, 10, 11 Routine cerebral imaging and electroencephalography were not available. Fundoscopic examination to look for retinal changes consistent with cerebral malaria (patchy retinal whitening, vessel colour changes, and white-centred haemorrhages) was possible in a subset of patients with deep coma with or without parasitaemia who were being investigated as part of another study of cerebral malaria.4 Children with parasitaemia were treated with oral sulfadoxine–pyrimethamine or parenteral quinine if they had features of severe malaria. No antiviral drugs were available.
Blood was obtained on admission for haematocrit measurement, blood cultures, examination for asexual forms of P falciparum on thick blood film (with a simplified WHO method12), and HIV testing after counselling at the discretion of the treating clinician (with Uni-Gold [Trinity Biotech, Bray, Ireland] and Determine [Abbott Laboratories, Green Oaks, IL, USA])·
All CSF specimens were stored at −80°C before processing. With previously described methods, we extracted DNA and did PCR to detect adenovirus,13 cytomegalovirus,14 Epstein-Barr virus,15 herpes simplex virus 1 and 2,16 human herpes virus 6 and 7,14 JC/BK virus,17 parvovirus B19,18 and varicella zoster virus. We identified adenovirus subgroups with restriction endonuclease analysis. We extracted RNA from CSF with the QIAamp Viral RNA Mini Kit (QIAGEN, Crawley, UK) and did PCR for mumps virus, enteroviruses, and parechoviruses.19 For measles virus, we designed primer probes for the haemagglutinin gene region with ABI Primer Express (Applied Biosystems, UK). We did diagnostic investigations for rabies on brain biopsy specimens from children who died, as described previously.20
Statistical analysis
We compared clinical features of the patients who died and those who survived with Fischer's exact and Kruskal-Wallis tests, as appropriate. We assessed factors associated with mortality with logistic regression. Several variables were of interest, such as sex, age, vomiting, hepatomegaly, splenomegaly, and HIV status. We did both univariate and multivariate logistic regression. We report odds ratios with 95% CIs. We used the 5% significance level. All analyses were done in SPSS (version 12.0) and Amos (version 6.0).
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
1230 children were admitted to hospital with suspected CNS infections, of whom 516 were deemed to have suspected viral CNS infection (figure). Three were excluded because of incomplete data, leaving 513 chidren. Median age was 2 years (table 1).
Figure.
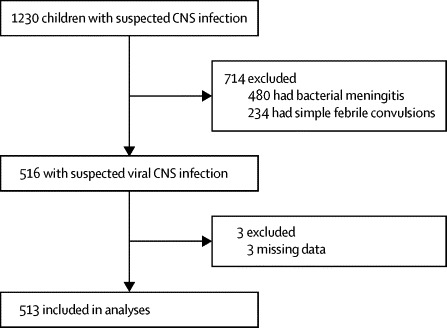
Trial profile
Table 1.
Clinical features at presentation and initial investigations by outcome
| All (n=513) | Survived (n=419) | Died (n=94) |
Odds ratio (95% CI)* |
|||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| Characteristics | ||||||
| Male sex | 281 (55%) | 225 (54%) | 56 (60%) | 1·3 (0·8–2·0) | 1·2 (0·6–2·0) | |
| Age (months) | 24 (7–51) | 24 (7–48) | 24 (9–72) | .. | .. | |
| Seizures | 204 (40%) | 161 (38%) | 43 (46%) | 1·3 (0·9–2·1) | 2·3 (0·7–8·1) | |
| Unconsciousness | 121 (24%) | 91 (22%) | 30 (32%) | 1·7 (1·0–2·7)† | 1·0 (0·4–2·3) | |
| Fever ≥3 days | 188 (37%) | 153 (37 %) | 35 (37%) | .. | .. | |
| Vomiting | 70 (14%) | 57 (14 %) | 13 (14%) | 1·3 (0·6–2·8) | .. | |
| Diarrhoea | 40 (8%) | 32 (8%) | 8 (8%) | 1·4 (0·6–3·3) | .. | |
| Runny nose | 41 (8%) | 35 (8 %) | 6 (6%) | 1·0 (0·4–2·7) | .. | |
| Headache | 36 (7%) | 33 (8%) | 3 (3%) | 0·9 (0·6–1·4) | .. | |
| Examination | ||||||
| Temperature ≥39°C | 48 (9%) | 39 (9%) | 9 (10%) | 1·0 (0·5–2·2) | 0·4 (0·1–1·3) | |
| Deep coma | 144 (28%) | 93 (22%) | 51 (54%) | 4·2 (2·6–6·6)† | 6·7 (3·3–13·8)‡ | |
| Bulging fontanelle | 12 (2%) | 7 (2%) | 5 (5%) | 3·3 (1·0–10·6) | 1·5 (0·4–6·8) | |
| Neck stiffness | 39 (8%) | 31 (7%) | 8 (9%) | 1·2 (0·5–2·6) | 1·5 (0·5–4·7) | |
| Abnormal chest | 26 (5%) | 18 (4%) | 8 (9%) | 2·1 (1·1–3·8)† | 1·6 (0·7–3·8) | |
| Hepatomegaly | 55 (11%) | 43 (10 %) | 12 (13%) | 1·6 (0·7–3·5) | .. | |
| Splenomegaly | 54 (11%) | 47 (11%) | 7 (7%) | 0·7 (0·3–1·6) | .. | |
| Hepatosplenomegaly | 36 (7%) | 31 (7%) | 5 (5%) | 0·8 (0·3–2·1) | .. | |
| Flexor posturing | 9 (2%) | 5 (1%) | 4 (4 %) | 3·7 (1·0–13·9) | 6·7 (0·8–59·3) | |
| Extensor posturing | 6 (1%) | 5 (1 %) | 1 (1%) | 0·9 (0·1–7·7) | 0·2 (0·0–4·0) | |
| Opisthotonus | 12 (2%) | 9 (2%) | 3 (3%) | 1·5 (0·4–5·6) | 0·8 (0·1–6·5) | |
| Observed seizure | 19 (4%) | 13 (3%) | 6 (6%) | 2·0 (0·7–5·2) | 1·4 (0·3–5·7) | |
| Investigations | ||||||
| CSF opening pressure ≥230 cm | 8 (2%) | 3 (1%) | 5 (5%) | 7·8 (1·8–33·0)† | 18·0 (2·7–121·6)‡ | |
| CSF white cell count >5 per μL | 188 (37%) | 152 (36%) | 36 (38%) | 1·4 (0·9–2·4) | 1·6 (0·9–2·8) | |
| HIV status | ||||||
| Positive | 49 (10%) | 32 (8%) | 17 (18%) | 6·6 (2·5–17·4)† | 17·1 (4·3–67·3)‡ | |
| Negative | 91 (18%) | 84 (20%) | 7 (7%) | .. | .. | |
| Not known | 373 (73%) | 303 (72 %) | 70 (74%) | 2·8 (1·2–6·3)† | 8·6 (2·4–30·9)‡ | |
| Malaria parasitaemia and CNS virus | 45 (9%) | 28 (7%) | 17 (18%) | 4·0 (2·0–8·1) | 1·9 (0·7–5·2) | |
| Malaria parasitaemia and no CNS virus | 118 (23%) | 101 (24%) | 17 (18%) | 1·1 (0·6-2·1) | 0·8 (0·4–1·8) | |
| CNS virus but no malaria parasitaemia | 88 (17%) | 62 (15%) | 26 (28%) | 2·8 (1·6–5·0) | 2·3 (1·1–4·9) | |
| No malaria parasitaemia or CNS virus | 260 (51%) | 226 (54%) | 34 (36%) | 1 | 1 | |
Data are n (%) or median (IQR), unless otherwise stated. CSF=cerebrospinal fluid.
Comparison of children who survived and died.
Significant on univariate analysis.
Significant on multivariate analysis.
A substantial proportion of children had a history of seizures (table 1). 144 (28%) presented in deep coma. More than a third had CSF pleocytosis (median white cell count 18 cells per mm3, IQR 10–60; table 1).
94 children (18%) died. 363 (71%) made apparent full recoveries, and 56 (11%) had neurological sequelae at discharge (including motor and cognitive deficits). Univariate analysis showed that a history of unconsciousness, deep coma on admission, abnormal findings on chest examination, and high CSF opening pressure were associated with a fatal outcome (table 1). However, on multivariate analysis, only deep coma and high CSF opening pressure were independently predictive of death (table 1). HIV status was established for 140 children (27%), of whom 49 (35%) were HIV positive. In both multivariate and univariate analysis, a negative HIV test was associated with survival, but a positive test or no test was associated with death (table 1).
163 children (32%) had P falciparum parasitaemia, of whom 34 (21%) died compared with 60 (17%) of 348 without parasitaemia (p=0·12). We recorded no association between the density of parasitaemia and outcome (data not shown). Children with parasitaemia were significantly older (median age 31 months, IQR 2–156) than were those without parasitaemia (18 months, 1–168; p<0·0001), and were more likely to present in deep coma (78 [48%] vs 66 [19%]; p<0·0001). 58 children with malaria parasitaemia (36%) had an HIV test, of whom 14 (24%) were HIV positive. Fundoscopy was done in 90 children. 44 children (49%) had parasitaemia, of whom 20 (45%) had evidence of malaria retinopathy. None of 46 patients without parasitaemia had retinopathy.
At least one virus was detected in the CNS in 133 children (26%). The most commonly detected virus was adenovirus, followed by mumps virus, human herpes virus 6, and rabies virus (table 2). 43 children with a CNS virus (33%) died compared with 51 (13%) of 378 with no CNS virus detected (p<0·0001; table 1). Seizures and deep coma were common in children with a CNS virus (table 2). The highest mortality was for rabies virus, followed by cytomegalovirus (table 2).
Table 2.
Clinical features and initial investigations by virus
| Adenovirus (n=42) | Cytomegalovirus (n=12) | Enterovirus (n=8) | Herpes simplex virus 1 (n=8) | Human herpes virus 6 (n=16) | Mumps virus (n=29) | Rabies virus (n=14) | Other virus (n=14)* | No virus (n=380) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | ||||||||||
| Male sex | 22 (52%) | 7 (58%) | 6 (75%) | 7 (88%) | 12 (75%) | 15 (52%) | 11 (79%) | 8 (57%) | 193 (51%) | |
| Age (months) | 15 (6–48) | 12 (3–15) | 21 (9–26) | 27 (16–51) | 24 (9–42) | 30 (7–78) | 84 (72–132) | 36 (13–72) | 23 (8–48) | |
| Seizures | 19 (45%) | 5 (42%) | 3 (38%) | 3 (38%) | 9 (56%) | 8 (28%) | 6 (43%) | 10 (71%) | 141 (37%) | |
| Unconsciousness | 3 (7%) | 4 (33%) | 1 (13%) | 3 (38%) | 7 (44%) | 8 (28%) | 5 (36%) | 6 (43%) | 84 (22%) | |
| Fever ≥3 days | 16 (38%) | 1 (8%) | 3 (38%) | 3 (38%) | 6 (38%) | 10 (34%) | 0 | 6 (43%) | 143 (38%) | |
| Vomiting | 3 (7%) | 1 (8%) | 1 (13%) | 4 (50%) | 5 (31%) | 4 (14%) | 0 | 1 (7%) | 51 (13%) | |
| Diarrhoea | 4 (10%) | 2 (17%) | 1 (13%) | 0 | 2 (13%) | 1 (3%) | 0 | 1 (7%) | 29 (8%) | |
| Runny nose | 6 (14%) | 2 (17%) | 2 (25%) | 1 (13%) | 3 (19%) | 1 (3%) | 0 | 2 (14%) | 24 (6%) | |
| Headache | 2 (5%) | 0 | 1 (13%) | 3 (38%) | 2 (13%) | 2 (7%) | 0 | 0 | 26 (7%) | |
| Examination | ||||||||||
| Temperature ≥39°C | 3 (7%) | 1 (8%) | 0 | 0 | 1 (6%) | 1 (3%) | 2 (14%) | 1 (7%) | 39 (10%) | |
| Deep coma | 12 (29%) | 6 (50%) | 1 (13%) | 3 (38%) | 7 (44%) | 9 (31%) | 4 (29%) | 5 (36%) | 101 (27%) | |
| Bulging fontanelle | 1 (2%) | 2 (17%) | 0 | 1 (13%) | 3 (19%) | 0 | 0 | 0 | 5 (1%) | |
| Neck stiffness | 2 (5%) | 4 (33%) | 0 | 0 | 1 (6%) | 1 (3%) | 4 (29%) | 1 (7%) | 26 (7%) | |
| Abnormal chest | 6 (14%) | 2 (17%) | 1 (13%) | 1 (13%) | 2 (13%) | 0 | 0 | 2 (14%) | 12 (3%) | |
| Hepatomegaly | 4 (10%) | 1 (8%) | 2 (25%) | 3 (38%) | 3 (19%) | 2 (7%) | 0 | 1 (7%) | 39 (10%) | |
| Splenomegaly | 3 (7%) | 0 | 1 (13%) | 3 (38%) | 2 (13%) | 3 (10%) | 0 | 1 (7%) | 41 (11%) | |
| Hepatosplenomegaly | 3 (7%) | 0 | 1 (13%) | 2 (25%) | 2 (13%) | 1 (3%) | 0 | 1 (7%) | 27 (7%) | |
| Flexor posturing | 0 | 1 (8%) | 0 | 0 | 2 (13%) | 2 (7%) | 0 | 0 | 4 (1%) | |
| Extensor posturing | 0 | 0 | 0 | 0 | 1 (6%) | 1 (3%) | 0 | 0 | 4 (1%) | |
| Opisthotonus | 1 (2%) | 1 (8%) | 0 | 0 | 0 | 0 | 0 | 0 | 10 (3%) | |
| Observed seizure | 5 (12%) | 0 | 0 | 0 | 2 (13%) | 0 | 1 (7%) | 1 (7%) | 12 (3%) | |
| Investigations | ||||||||||
| CSF opening pressure ≥230 cm | 0 | 1 (8%) | 0 | 0 | 1 (6%) | 0 | 2 (14%) | 0 | 4 (1%) | |
| CSF white cell count >5 per μL | 11 (26%) | 1 (8%) | 4 (50%) | 2 (25%) | 0 | 11 (38%) | 2 (14%) | 8 (57%) | 149 (39%) | |
| HIV status | ||||||||||
| Positive | 0 | 2 (17%) | 1 (13%) | 0 | 2 (13%) | 2 (7%) | 0 | 2 (14%) | 40 (11%) | |
| Negative | 11 (26%) | 1 (8%) | 1 (13%) | 0 | 7 (44%) | 7 (24%) | 0 | 3 (21%) | 61 (16%) | |
| Not known | 29 (69%) | 6 (50%) | 5 (63%) | 0 | 6 (38%) | 19 (66%) | 14 (100%) | 10 (71%) | 284 (75%) | |
| Malaria parasitaemia and CNS virus | 11 (26%) | 3 (25%) | 0 | 2 (25%) | 5 (31%) | 7 (24%) | 5 (36%) | 8 (57%) | .. | |
| CNS virus but no malaria parasitaemia | 26 (62%) | 5 (42%) | 5 (63%) | 5 (63%) | 7 (44%) | 19 (66%) | 9 (64%) | 6 (43%) | .. | |
| Outcome | ||||||||||
| Sequelae | 4 (10%) | 2 (17%) | 2 (25%) | 0 | 2 (13%) | 3 (10%) | 0 | 2 (14%) | 39 (10%) | |
| Died | 11 (26%) | 10 (83%) | 3 (38%) | 1 (13%) | 1 (6%) | 5 (17%) | 14 (100%) | 3 (21%) | 46 (12%) | |
Data are n (%) or median (IQR). 11 patients (not shown here) had dual virus infections; each patient had a different combination of viruses: adenovirus in five cases, human herpes virus in four, cytomegalovirus in four, enterovirus in three, mumps in three, herpes simplex virus in one, measles in one, and parvovirus in one. Genotyping indicated that seven of the adenoviruses detected were members of subgroup B, one was subgroup C. Median age of patients with dual infection was 18 months (IQR 15–26), and Blantyre coma score on admission 3 (2–5). Seven patients with dual infection had malaria parasitaemia, three were HIV positive (of five tested), and three had a CSF pleocytosis.·Six children (55%) with dual viral infections died. CSF=cerebrospinal fluid.
Epstein-Barr virus (five patients), parvovirus (five), human herpes virus 7 (two), measles (one), JC/BK virus (one).
The median age of children with a viral CNS infection was 26 months (IQR 7–72) compared with 23 (8–48) for those with no virus (p=0·1843). Of the 41 children with a viral CNS infection who were tested for HIV, 12 (29%) were positive; of the 99 with no viral CNS infection who were tested for HIV, 37 (37%) were positive (p=0·0438).
To further examine the relation between malaria parasitaemia, viral CNS infection, and outcome, we divided the patients into four groups depending on their malaria and viral infection status (table 3). 45 (9%) of the 513 children had both a viral CNS infection and malaria parasitaemia. No specific virus was associated with parasitaemia (table 2). In an unadjusted comparison, we examined differences between the four groups in clinical presentation. The proportion of children with a history of seizures was highest for those with malaria and viral CNS infection, followed by those with malaria only, those with neither infection, and those with only viral infection (table 3). The four groups also differed significantly in the proportion with a history of unconsciousness and presenting in deep coma, both characteristics being most common in children with dual infection (table 3). 27 (35%) of 78 children who had malaria parasitaemia and deep coma (ie, fulfilled the clinical definition of cerebral malaria) also had a viral CNS infection (table 3).
Table 3.
Clinical features and initial investigation by malaria parasitaemia and viral CNS infection
|
Malaria parasitaemia |
No malaria parasitaemia |
p value* | ||||||
|---|---|---|---|---|---|---|---|---|
| CNS virus (n=45) | No CNS virus (n=118) | Unadjusted odds ratio (95% CI) | CNS virus (n=88) | No CNS virus (n=262) | Unadjusted odds ratio (95% CI) | |||
| Characteristics | ||||||||
| Male sex | 28 (62%) | 60 (51%) | 1·6 (0·7–3·4) | 52 (59%) | 141 (54%) | 1·2 (0·7–2·1) | 0·479 | |
| Age (months) | 34 (9–72) | 30 (15–50) | 1·0 (1·0–1·0) | 23 (6–78) | 14 (6–48) | 1·0 (1·0–1·0) | 0·001 | |
| Seizures | 32 (71%) | 51 (43%) | 3·2 (1·5–7·4) | 30 (34%) | 91 (35%) | 0·97 (0·6–1·7) | <0·0001 | |
| Generalised | 27 (60%) | 37 (31%) | 3·2 (1·5–7·1) | 25 (28%) | 72 (27%) | 1·0 (0·6–1·8) | <0·0001 | |
| Focal | 6 (13%) | 8 (7%) | 2·1 (0·6–7·4) | 3 (3%) | 15 (6%) | 0·6 (0·1–3·0) | 0·172 | |
| Previous history | 8 (18%) | 8 (7%) | 2·6 (0·8–8·0) | 6 (7%) | 19 (7%) | 1·1 (0·4–3·0) | 0·139 | |
| Unconsciousness | 23 (51%) | 44 (37%) | 2·1 (0·8–8·0) | 13 (15%) | 41 (16%) | 1·4 (0·6–3·0) | <0·0001 | |
| Fever ≥3 days | 16 (36%) | 48 (41%) | 1·6 (0·6–3·8) | 29 (33%) | 95 (36%) | 0·9 (0·5–1·8) | 0·719 | |
| Vomiting | 9 (20%) | 20 (17%) | 1·2(0·4–3·1) | 8 (9%) | 33 (13%) | 0·7 (0·3–1·6) | 0·208 | |
| Diarrhoea | 2 (4%) | 10 (8%) | 0·5 (0·1–2·5) | 8 (9%) | 20 (8%) | 1·2 (0·4–3·0) | 0·841 | |
| Runny nose | 6 (13%) | 5 (4%) | 3·5 (0·8–15·1) | 8 (9%) | 22 (8%) | 1·1 (0·4–2·7) | 0·209 | |
| Headache | 2 (4%) | 14 (12%) | 0·3 (0·0–1·6) | 8 (9%) | 12 (5%) | 1·1 (0·4–2·8) | 0·054 | |
| Examination | ||||||||
| Temperature ≥39°C | 3 (7%) | 19 (16%) | 0·6 (0·4–1·0) | 5 (6%) | 21 (8%) | 1·1 (0·8–1·6) | 0·022 | |
| Deep coma | 27 (60%) | 51 (43%) | 0·5 (0·3–1·0) | 16 (18%) | 50 (19%) | 1·1 (0·8–2·0) | <0·0001 | |
| Bulging fontanelle | 1 (2%) | 1 (1%) | 2·0 (0·1–78·2) | 5 (6%) | 5 (2%) | 3·1 (0·7–13·8) | 0·142 | |
| Neck stiffness | 1 (2%) | 4 (3%) | 0·5 (0·1–4·7) | 6 (7%) | 28 (11%) | 0·6 (0·2–1·6) | 0·044 | |
| Abnormal chest | 3 (7%) | 4 (3%) | 2·0 (0·3–12·5) | 9 (10%) | 10 (4%) | 2·9 (1·0–18·1) | 0·091 | |
| Hepatomegaly | 10 (22%) | 26 (22%) | 0·7 (0·3–2·0) | 5 (6%) | 14 (5%) | 1·1 (0·4–3·5) | <0·0001 | |
| Splenomegaly | 10 (22%) | 22 (19%) | 1·0 (0·4–2·7) | 3 (3%) | 19 (7%) | 0·4 (0·1–1·6) | <0·0001 | |
| Flaccid paralysis | 2 (4%) | 11 (9%) | 0·3 (0·1–1·5) | 1 (1%) | 7 (3%) | 0·4 (0·0–3·3) | <0·0001 | |
| Spastic paralysis | 4 (9%) | 5 (4%) | 2·0 (0·5–8·3) | 6 (7%) | 9 (3%) | 1·9 (0·5–6·8) | 0·442 | |
| Flexor posturing | 3 (7%) | 5 (4%) | 1·4 (0·3–6·3) | 0 | 1 (<1%) | .. | 0·002 | |
| Extensor posturing | 1 (2%) | 2 (2%) | 1·1 (0·1–12·8) | 0 | 3 (1%) | .. | 0·550 | |
| Opisthotonus | 0 | 4 (3%) | .. | 1 (1%) | 7 (3%) | 0·4 (0·0–3·3) | 0·661 | |
| Extensor plantar | 7 (16%) | 16 (14%) | 1·1 (0·4–3·3) | 8 (9%) | 24 (9%) | 1·4 (0·5–3·8) | 0·372 | |
| Observed seizure | 6 (13%) | 6 (5%) | 2·6 (0·7–9·2) | 2 (2%) | 7 (3%) | 0·9 (0·2–4·8) | 0·017 | |
| Investigations | ||||||||
| CSF opening pressure ≥230 cm | 2 (4%) | 5 (4%) | 1·2 (0·2–7·9) | 0 | 1 (<1%) | .. | 0·930 | |
| CSF white cell count >5 per μL | 11 (24%) | 48 (41%) | 0·5 (0·3–1·0) | 31 (35%) | 98 (37%) | 1·1 (0·6–1·8) | 0·501 | |
| HIV positive | 4 (9%) | 10 (8%) | 0·9 (0·2–3·2) | 8 (9%) | 27 (10%) | 0·6 (0·2–1·7) | 0·971 | |
| Outcome | ||||||||
| Died | 17 (38%) | 17 (14%) | 3·6 (1·6–8·0) | 26 (30%) | 34 (13%) | 2·8 (1·6–5·0) | <0·0001 | |
Data are n (%) or median (IQR), unless otherwise stated. CSF=cerebrospinal fluid.
Unadjusted comparison across all four groups.
The proportion of patients with flexor posturing and flaccid paralysis differed significantly between groups (table 3). Viruses were detected in the CSF of five (25%) of 20 children with parasitaemia and malaria retinopathy, two of whom died, compared with eight (33%) of 24 children with parasitaemia who had normal fundoscopy, none of whom died.
Outcome differed significantly between the four groups, with a fatal outcome most common in patients with parasitaemia and viral infection (table 3). Logistic regression analysis showed that children with a viral CNS infection had a significantly higher mortality than did those who did not have a viral CNS infection (p=0·001), but we recorded no significant difference in mortality between those who were positive or negative for malaria parasitaemia, irrespective of virus status (p=0·705)·
Discussion
We have shown that viral CNS infections are an important cause of hospital admission and death in Malawian children (panel). A third of the children who met the case definition for cerebral malaria had viral infections, including a quarter of the children who had the characteristic retinal changes thought to be diagnostic of cerebral malaria. Although fewer patients had viral infection than parasitaemia, more children with viral infection died, even when patients with rabies—which is uniformly fatal—were excluded.
Panel. Research in context.
Systematic review
We searched PubMed for studies of viral CNS infections in sub-Saharan Africa, with the terms “virus” and “CNS infection”, “meningitis”, or “encephalitis”. We included reports published in English between Jan 1, 1966, and Jan 1, 2013. We refined the search to exclude reports describing polio eradication. Although malaria and bacteria have been widely studied as causes of CNS disease in sub-Saharan Africa, little work on viral pathogens has been done. Previous studies have shown that enteroviruses, herpes simplex virus, and mumps could be important.5
Interpretation
We tested children with suspected non-bacterial CNS infection for 15 different viruses and have shown that viral CNS infections are much more common than previously suspected. We recorded a range of pathogens that is different from that typically reported in developed nations. Viral pathogens were identified in a third of comatose children with malaria parasitaemia who might otherwise have been diagnosed as having cerebral malaria. Children with dual viral and parasitic infection had more severe disease than did others, suggesting an interaction between the two. Importantly, viral infections overall were associated with more fatal cases than was malaria parasitaemia. These results have important implications for malaria control and management of patients with suspected CNS infections in sub-Saharan Africa.
The number of patients with viral CNS disease might have been even higher had we included children with suspected bacterial meningitis or simple febrile convulsions. However, in a study of 142 children with suspected bacterial meningitis,21 a virus was detected in the CSF of just one patient with more than 1000 white blood cells in the CSF.
A study of Kenyan children5 previously showed that ten of 96 children with encephalopathy were infected with herpes simplex virus, but only tests for this virus and enteroviruses were done. In our study, viruses were detected in the CNS of a quarter of 513 patients studied. Although this frequency is similar to that in studies from Europe, the Americas, and Asia,22, 23, 24 the range of infections was different. In developed countries, herpes simplex virus, varicella zoster virus, and enteroviruses are major causes of CNS infection; by contrast, we recorded that adenovirus was the most common virus, accounting for about a third of cases of viral CNS infection. Adenoviruses most often cause respiratory illness or conjunctivitis. In the past, when only serological tests were available, their role in causing neurological disease was unclear. With widespread use of CSF PCR, adenoviruses have been associated increasingly with a range of neurological syndromes, such as acute flaccid paralysis, meningitis, and encephalitis, sometimes with immunohistochemical confirmation.25, 26, 27 In our study, about a quarter of patients with adenovirus infection died, which is similar to the frequency in previous reports.28
Clearly, further work is needed to explore the role of adenoviruses in CNS disease in this setting. Few large series examining CNS infections have been focused on adenoviruses and, as far as we are aware, we are the first to do so in sub-Saharan Africa. Whether specific features make individuals in this region particularly susceptible to adenovirus CNS infection—eg, immunodeficiency due to HIV, poor nutritional state, malaria, or anaemia—will need to be investigated.
The high frequency of mumps encephalitis in our study was probably a result of poor routine vaccination; the virus's importance in aseptic meningitis has been shown previously in South Africa.29 By contrast, only one child had measles encephalitis in our study, which is a result of successful vaccination. Human herpes virus 6 is being recognised increasingly as a cause of encephalitis in children in all settings,30 although false positives can occur because of chromosomal integration.31 CNS infection of the brain with cytomegalovirus is especially common in people who are HIV positive; in our study, more than 40% of children with cytomegalovirus infection were HIV positive, and many of them also had an additional virus detected in their CSF. Since our study was done, antiretroviral therapy for HIV has become increasingly available in Malawi. Although we recorded some differences in the clinical features at presentation for the different viruses—eg, hydrophobia for patients with rabies and a high proportion of patients with human herpes virus 6 affected by seizures—clinical features could, for the most part, not be used to distinguish between the different viral infections.
We focused on the CNS, but had we also examined blood, throat, and rectal swabs, we might have detected viruses in more patients than we report here. However, if we had examined such samples, we would then have had to try to establish whether any identified peripheral infection was causal or not.32, 33 Antibody testing of CSF with paired blood samples might also have been helpful, but CSF volumes were small, and so, as with previous studies, we opted for the strictest criteria to define CNS infections, concentrating on virus detection in the CNS itself.22 Many children were negative for both malaria parasites and viral pathogens. They tended to be younger than those who were positive for either, but had no other distinguishing features.
Without our detailed investigation, the altered consciousness of children who met the case definition for cerebral malaria would have been attributed solely to malaria. Do such patients have a viral CNS infection and coincidental malaria parasitaemia? Asymptomatic parasitaemia certainly does occur, and coincidental P falciparum infection is recognised in patients with bacterial meningitis.3 Alternatively, could patients have a coincidental viral infection? Although detection of viruses outside the CNS, particularly from non-sterile sites such as the rectum, might be coincidental,32 virus detected within the CNS is usually deemed to be pathogenic, especially if it is associated with a recognised neurological syndrome. Exceptions are some of the herpes viruses: human herpes virus 6 is known to integrate within leucocytes in about 1% of the population, and thus might be coincidentally detected. Additionally, Epstein-Barr virus and cytomegalovirus lie latent within lymphocytes; their role in encephalitis and other CNS diseases is recognised increasingly in both immunocompetent and immunocompromised patients.34, 35 Quantification of viral loads might have provided additional information in our study, but at the time was not routinely available for all viruses studied. However, Epstein-Barr virus is often detected in the CSF of patients with HIV and bacterial meningitis, and higher loads are associated with a worse outcome.36 In other studies, Epstein-Barr virus messenger RNA from the replication cycle has been detected in the CSF of patients with CNS infections,37 and production of high titres of intrathecal antibodies34 in such patients has been shown, all of which is consistent with Epstein-Barr virus replicating and causing disease, rather than simply lying latent in lymphocytes.
A post-mortem study38 showed that 23% of children in Malawi who fulfilled the WHO definition of cerebral malaria died of other causes. Retinopathy was the only clinical sign that could be used to distinguish between children with cerebral sequestration of parasitised erythrocytes and those without sequestration. In that study,38 the other causes included Reye's syndrome, ruptured arteriovenous malformation, and hepatic necrosis, but detailed virological work-up was not done as in our study. We noted that viral CNS infection was equally common in children with parasitaemia with or without retinal changes of cerebral malaria, which suggests that some of our patients could have had viral CNS infection with coincidental parasitaemia, while others had viral CNS infection and cerebral malaria, as defined by fundoscopy. Although the numbers were small, there was no difference in mortality between children with and without malaria retinopathy. This finding agrees with another study39 that showed similar outcomes for children meeting the clinical case definition for cerebral malaria, whether or not they had retinopathy.
Different viruses have different clinicoepidemiological patterns and disease mechanisms. However, if they are grouped together, as is done for bacteria in bacteraemia studies, overall disease burden can be examined. We recorded that children with malaria parasitaemia and viral co-infection were more likely to present with seizures than were those with either infection alone or neither infection, raising the possibility that both pathogens contribute to the pathogenesis in patients with dual infections. How viruses and malaria parasites might interact is unknown. In studies of sepsis and malaria, the effect of microvascular parasite sequestration on the integrity of the gut mucosa is thought to allow bacterial seeding into the blood stream and hence bacteraemia.40 A similar effect, disrupting the endothelial cell junctional proteins of the blood–brain barrier,41 might damage the barrier and allow virus into the CNS; in which case, detection of virus in the CSF could be a result of this disruption, with the virus playing no part in pathogenesis. Additionally, viruses are known to trigger upregulation of vascular endothelial adhesion molecules, such as ICAM-1,42 which might lead to increased sequestration of parasitised erythrocytes, thus exacerbating malaria-related cerebral injury. The interactions between different pathogens and their aggregate effect on an infected individual are incompletely understood and poorly investigated.43
Acknowledgments
Acknowledgments
MMa was supported by a Wellcome Trust Fellowship (058390/Z/99/Z). TT was supported by the US National Institutes of Health (5R01AI034969-14). TS was supported by a UK Medical Research Council Senior Clinical Fellowship (G116/194). We thank the children and their parents for participating in the study; the staff of Queen Elizabeth Hospital Blantyre for their support; Tony Fooks for helpful discussions on the diagnostic rabies virology; and Alister Craig for review.
Contributors
MMa, MMo, and TS had the original idea for the study. MMa, PV, BF, DB, MMu, HK, PP, TT, Mmo, and TS designed and implemented the study, and gathered data and samples. PV and PK were primarily responsible for the virological studies. PV devised and designed viral laboratory techniques and supervised laboratory work. PK supervised laboratory work and quality assurance of PCR techniques. MMa and TS wrote the first draft of the report, with input from PV, BF, PK, and TT. All authors approved the final version.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.WHO . Management of severe malaria—a practical handbook. World Health Organization; Geneva: 2000. [Google Scholar]
- 2.Snow RW, Omumbo JA, Lowe B. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 3.Berkley JA, Mwangi I, Mellington F, Mwarumba S, Marsh K. Cerebral malaria versus bacterial meningitis in children with impaired consciousness. Q J Med. 1999;93:151–157. doi: 10.1093/qjmed/92.3.151. [DOI] [PubMed] [Google Scholar]
- 4.Beare NA, Lewallen S, Taylor TE, Molyneux ME. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol. 2011;6:349–355. doi: 10.2217/fmb.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubart CD, Mturi N, Beld MG, Wertheim PM, Newton CR. Role of viruses in Kenyan children presenting with acute encephalopathy in a malaria-endemic area. Am J Trop Med Hyg. 2006;75:1148–1150. [PubMed] [Google Scholar]
- 6.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 7.Nelson KB, Ellenberg JH. Prognosis in children with febrile seizures. Pediatrics. 1978;61:720–727. [PubMed] [Google Scholar]
- 8.Molyneux EM, Walsh AL, Forsyth H. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomised controlled trial. Lancet. 2002;360:211–218. doi: 10.1016/s0140-6736(02)09458-8. [DOI] [PubMed] [Google Scholar]
- 9.McCormick DW, Wilson ML, Mankhambo L. Risk factors for death and severe sequelae in Malawian children with bacterial meningitis, 1997–2010. Pediatr Infect Dis J. 2013;32:e54–e61. doi: 10.1097/INF.0b013e31826faf5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 11.Newton CR, Kirkham FJ, Johnston B, Marsh K. Inter-observer agreement of the assessment of coma scales and brainstem signs in non-traumatic coma. Dev Med Child Neurol. 1995;37:807–813. doi: 10.1111/j.1469-8749.1995.tb12064.x. [DOI] [PubMed] [Google Scholar]
- 12.Hommel M. In: Essential Malariology. 4th edn. Warrell DA, Gilles HM, editors. Edward Arnold; London: 2002. Diagnostic methods in malaria; pp. 35–56. [Google Scholar]
- 13.Cooper RJ, Yeo AC, Bailey AS, Tullo AB. Adenovirus polymerase chain reaction assay for rapid diagnosis of conjunctivitis. Invest Ophthalmol Vis Sci. 1999;40:90–95. [PubMed] [Google Scholar]
- 14.Ashshi AM, Klapper PE, Cooper RJ. Detection of human cytomegalovirus, human herpesvirus type 6 and human herpesvirus type 7 in urine specimens by multiplex PCR. J Infect. 2003;47:59–64. doi: 10.1016/s0163-4453(03)00057-4. [DOI] [PubMed] [Google Scholar]
- 15.Miyashita EM, Yang B, Lam KM, Crawford DH, Thorley-Lawson DA. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- 16.Orle KA, Weiss JB. Detection of Treponema pallidum, Haemophilus ducreyi, and herpes simplex virus by multiplex PCR. Methods Mol Med. 1999;20:67–79. doi: 10.1385/0-89603-535-2:67. [DOI] [PubMed] [Google Scholar]
- 17.Arthur RR, Dagostin S, Shah KV. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol. 1989;27:1174–1179. doi: 10.1128/jcm.27.6.1174-1179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durigon EL, Erdman DD, Gary GW. Multiple primer pairs for polymerase chain reaction (PCR) amplification of human parvovirus B19 DNA. J Virol Methods. 1993;44:155–165. doi: 10.1016/0166-0934(93)90051-r. [DOI] [PubMed] [Google Scholar]
- 19.Corless CE, Guiver M, Borrow R. Development and evaluation of a ‘real-time’ RT-PCR for the detection of enterovirus and parechovirus RNA in CSF and throat swab samples. J Med Virol. 2002;67:555–562. doi: 10.1002/jmv.10138. [DOI] [PubMed] [Google Scholar]
- 20.Mallewa M, Fooks AR, Banda D. Rabies encephalitis in malaria-endemic area, Malawi, Africa. Emerg Infect Dis. 2007;13:136–139. doi: 10.3201/eid1301.060810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelkonen T, Roine I, Anjos E. Picornaviruses in cerebrospinal fluid of children with meningitis in Luanda, Angola. J Med Virol. 2012;84:1080–1083. doi: 10.1002/jmv.23304. [DOI] [PubMed] [Google Scholar]
- 22.Granerod J, Ambrose HE, Davies NW. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 23.Le VT, Phan TQ, Do QH. Viral etiology of encephalitis in children in southern Vietnam: results of a one-year prospective descriptive study. PLoS Negl Trop Dis. 2010;4:e854. doi: 10.1371/journal.pntd.0000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser CA, Gilliam S, Schnurr D. In search of encephalitis etiologies: diagnostic challenges in the California encephalitis project, 1998–2000. Clin Infect Dis. 2003;36:731–742. doi: 10.1086/367841. [DOI] [PubMed] [Google Scholar]
- 25.Chuang YY, Chiu CH, Wong KS. Severe adenovirus infection in children. J Microbiol Immunol Infect. 2003;36:37–40. [PubMed] [Google Scholar]
- 26.Lee TC, Tsai CP, Yuan CL. Encephalitis in Taiwan: a prospective hospital-based study. Jpn J Infect Dis. 2003;56:193–199. [PubMed] [Google Scholar]
- 27.Dubberke ER, Tu B, Rivet DJ. Acute meningoencephalitis caused by adenovirus serotype 26. J Neurovirol. 2006;12:235–240. doi: 10.1080/13550280600846633. [DOI] [PubMed] [Google Scholar]
- 28.Simila S, Jouppila R, Salmi A, Pohjonen R. Encephaloningitis in children associated with an adenovirus type 7 epidemic. Acta Paediatr Scand. 1970;59:310–316. doi: 10.1111/j.1651-2227.1970.tb09009.x. [DOI] [PubMed] [Google Scholar]
- 29.McIntyre JP, Keen GA. Laboratory surveillance of viral meningitis by examination of cerebrospinal fluid in Cape Town, 1981–9. Epidemiol Infect. 1993;111:357–371. doi: 10.1017/s095026880005706x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao K, Crawford JR, Komaroff AL, Ablashi DV, Jacobson S. Review part 2: Human herpesvirus-6 in central nervous system diseases. J Med Virol. 2010;82:1669–1678. doi: 10.1002/jmv.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellett PE, Ablashi DV, Ambros PF. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol. 2012;22:144–155. doi: 10.1002/rmv.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9:1097–1105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 33.Ooi MH, Solomon T, Podin Y. Evaluation of different clinical sample types in the diagnosis of human enterovirus 71 associated hand-foot-and-mouth disease. J Clin Microbiol. 2007;45:1858–1866. doi: 10.1128/JCM.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleines M, Schiefer J, Stienen A. Expanding the spectrum of neurological disease associated with Epstein-Barr virus activity. Eur J Clin Microbiol Infect Dis. 2011;30:1561–1569. doi: 10.1007/s10096-011-1261-7. [DOI] [PubMed] [Google Scholar]
- 35.Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. doi: 10.1186/1743-422X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly MJ, Benjamin LA, Cartwright K. Epstein-Barr virus coinfection in cerebrospinal fluid is associated with increased mortality in Malawian adults with bacterial meningitis. J Infect Dis. 2012;205:106–110. doi: 10.1093/infdis/jir707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg A, Bloch KC, Li S. Dual infections of the central nervous system with Epstein-Barr virus. J Infect Dis. 2005;191:234–237. doi: 10.1086/426402. [DOI] [PubMed] [Google Scholar]
- 38.Taylor TE, Fu WJ, Carr RA. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 39.Postels DG, Taylor TE, Molyneux M. Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology. 2012;79:1268–1272. doi: 10.1212/WNL.0b013e31826aacd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott JA, Berkley JA, Mwangi I. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378:1316–1323. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria: a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CH, Huang Y, Issekutz AC. Interleukin-1alpha released from epithelial cells after adenovirus type 37 infection activates intercellular adhesion molecule 1 expression on human vascular endothelial cells. J Virol. 2002;76:427–431. doi: 10.1128/JVI.76.1.427-431.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obaro S, Greenwood B. Malaria and bacteraemia in African children. Lancet. 2011;378:1281–1282. doi: 10.1016/S0140-6736(11)61146-X. [DOI] [PubMed] [Google Scholar]


