Abstract
Intracellular free Ca2+ ([Ca2+]i) is a highly versatile second messenger that regulates a wide range of functions in every type of cell and tissue. To achieve this versatility, the Ca2+ signaling system operates in a variety of ways to regulate cellular processes that function over a wide dynamic range. This is particularly well exemplified for Ca2+ signals in the liver, which modulate diverse and specialized functions such as bile secretion, glucose metabolism, cell proliferation, and apoptosis. These Ca2+ signals are organized to control distinct cellular processes through tight spatial and temporal coordination of [Ca2+]i signals, both within and between cells. This article will review the machinery responsible for the formation of Ca2+ signals in the liver, the types of subcellular, cellular, and intercellular signals that occur, the physiological role of Ca2+ signaling in the liver, and the role of Ca2+ signaling in liver disease.
Introduction
Many of the functions of the liver are regulated by increases in intracellular Ca2+ ([Ca2+]i; a list of abbreviations used in this article is provided in Table 1). This includes vesicular trafficking and canalicular contraction, which are regulated by cytosolic Ca2+ ([Ca2+]c) and participate in the control of bile secretion (252, 398, 400, 415); glucose and energy metabolism, which occur through the modulation of regulatory enzyme activity (35, 109, 200) and through changes in mitochondrial Ca2+ ([Ca2+]m) (154, 326); and control of the cell cycle, which includes modulation of transcription (157, 314) and anti- and proapoptotic proteins (17, 221, 258) to regulate cell proliferation and death, and is regulated by nucleoplasmic Ca2+ ([Ca2+]n) (332). To control, yet discriminate among so many functions simultaneously, [Ca2+]i signals are highly organized in time (e.g., through the amplitude of Ca2+ spikes and the frequency of Ca2+ oscillations) and space (e.g., through Ca2+ gradients and waves). These specialized features of Ca2+ signals are mediated by the PLC/InsP3/InsP3R cascade, which may be activated by Ca2+ mobilizing hormones or growth factors and leads to release of Ca2+ stored in the endoplasmic reticulum (ER) (24, 25, 27, 72, 105, 379, 381). The interplay between different organelles in liver cells also determines the proper spatio-temporal modulation of Ca2+ signals and the execution of specific functions (310, 326, 343). In addition, Ca2+ signaling patterns are not just organized within individual cells but are coordinated among cells as well, through a complex communication network dependent on signaling through gap junctions (105, 266, 344) and paracrine messengers (104, 115, 259, 351) that extends across the hepatic lobule. This article describes the basic components required for the formation of Ca2+ signals in the liver, the mechanisms of distinct Ca2+ signaling pattern formation, and their involvement in processes related to health and disease.
Table 1.
List of Abbreviations
| [Ca2+]c | Cytosolic calcium |
| [Ca2+]i | Intracellular calcium |
| [Ca2+]m | Mitochondrial calcium |
| [Ca2+]n | Nucleoplasmic calcium |
| 5-FU | 5-fluorouracil |
| Ach | Acetylcholine |
| ADP | Adenosine diphosphate |
| AP-1 | Activator protein 1 |
| ATP | Adenosine triphosphate |
| ATF6α | Activating transcription factor 6α |
| AVP | Arginine vasopressin |
| BDL | Bile duct ligation |
| BSEP | Bile salt export pump |
| cADPR | Cyclic adenosine diphosphate ribose |
| CaMK | Calcium-calmodulin-dependent protein kinase |
| cAMP | Cyclic adenosine monophosphate |
| CD38 | Adenosine diphosphate-ribosyl cyclase |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CGamF | Cholylglycylamido-fluorescein |
| CICR | Calcium-induced calcium release |
| c-Met | Hepatocyte growth factor receptor |
| CMFDA | 5-chloromethylfluorescein diacetate |
| cPKCα | Ca2+-dependent conventional protein kinase C alpha |
| CRAC | Calcium release activated channel |
| CREB | Cyclic adenosine monophosphate response element binding |
| CsA | Cyclosporin A |
| Cx26 | Connexin 26 |
| Cx32 | Connexin 32 |
| DAG | Diacylglycerol |
| DMSO | Dimethylsulfoxide |
| EGF | Epidermal growth factor |
| Elk1 | E twenty-six-like transcription factor 1 |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FLZ | N-(2-(4-hydroxy-phenyl)-ethyl]-2-(2,5-dimethoxy-phenyl)-3-(3-methoxy-4-hydroxy-phenyl)-acrylamide |
| FXR | Farnesoid X receptor |
| GDP | Guanosine diphosphate |
| GFP | Green fluorescent protein |
| GPCR | G-protein-coupled receptor |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular Carcinoma |
| HCV | Hepatitis C Virus |
| HGF | Hepatocyte growth factor |
| HSC | Hepatic stellate cells |
| IFNα | Interferon-alpha |
| IGF-1 | Insulin-like growth factor 1 |
| IMM | Inner mitochondrial membrane |
| INM | Inner nuclear membrane |
| InsP3 | Inositol-1,4,5-trisphosphate |
| InsP3R | Inositol-1,4,5-trisphosphate receptor |
| IRE1α | Inositol requiring enzyme 1α |
| JNK | c-jun-N-terminal kinase |
| LCA | Lithocholic acid |
| LGMN | Legumain |
| LPS | Lipopolysaccharide |
| mCICR | Mitochondrial calcium induced calcium release |
| MCU | Mitochondrial calcium uniporter |
| MRP2 | Multidrug resistance associated protein 2 |
| NAADP | Nicotinic acid adenine dinucleotide phosphate |
| NAD(P)H | Nicotinamide adenine dinucleotide phosphate |
| NAFLD | Non-alcoholic fatty liver disease |
| NE | Nuclear envelope |
| NFAT | Nuclear factor of activated T-cells |
| NFKB | Nuclear factor kappa B |
| NPC | Nuclear pore complex |
| NR | Nucleoplasmic reticulum |
| OAP | Oct-1 associated protein |
| OMM | Outer mitochondrial membrane |
| ONM | Outer nuclear membrane |
| PDGF | Platelet-derived growth factor |
| PERK | Protein kinase ribonucleic acid-like endoplasmic reticulum kinase |
| PH | Partial hepatectomy |
| PIP2 | Phosphotidylinositol-4-5-bisphosphate |
| PKA | Cyclic adenosine monophosphate-dependent protein kinase |
| PKC | Protein kinase C |
| PKG | Cyclic guanosine monophosphate-dependent protein kinase |
| PLC | Phospholipase C |
| PT | Permeability transition |
| PTB | Phosphotyrosine binding |
| PTP | Permeability transition pore |
| PV | Parvalbumin |
| RaM | Rapid uptake mode |
| RASAL | Ras guanosine triphosphatase activating protein |
| RaSH | Rapid Subtraction Hybridization |
| ROS | Reactive oxygen species |
| RTK | Receptor Tyrosine Kinase |
| RyR | Ryanodine receptor |
| SERCA | Sarco/endoplasmic reticulum calcium adenosine triphosphatase |
| SH | Src homology domain |
| SOC | Store-operated calcium channel |
| STIM1 | Stromal interaction factor 1 |
| TBH | Tertbutylhydroperoxide |
| tBHP | Tert-butyl hydroperoxide |
| TUDCA | Tauroursodeoxycholic acid |
| TLCA | Taurolithocholic acid |
| TPC | Two-pore channel |
| TRPV | Transient receptor potential vanilloid |
| UDCA | Ursodeoxycholic acid |
| UPR | Unfolded protein response |
| VDAC | Voltage-dependent anion channel |
General Molecular and Cellular Mechanisms of Ca2+ Signaling
Molecular machinery for Ca2+ signal formation in the cytoplasm
The Ca2+ signaling system contains a wide molecular set of signaling components that act in synergy to drive Ca2+ signals with unique spatial and temporal properties (25, 28). [Ca2+]c signals can be derived from the extracellular medium or from internal stores. In the case of the latter, several intracellular messengers bind to and regulate the activity of specific intracellular receptors to promote Ca2+ release (25, 41). Inositol-1,4,5-trisphosphate (InsP3) is one such Ca2+-mobilizing messenger and acts through the InsP3 Receptor (InsP3R). Similarly, Ca2+ itself and cyclic ADP ribose (cADPR) promote [Ca2+]c signals through their interaction with ryanodine receptors (RyRs). Additionally, nicotinic acid adenine dinucleotide phosphate (NAADP) activates two-pore channels (TPCs) (25, 28, 41, 58, 178).
InsP3-mediated Ca2+ release is initiated by the binding of Ca2+ mobilizing hormones or growth factors to plasma membrane G-protein-coupled receptors (GPCRs) (23, 25, 357) or receptor tyrosine kinases (RTKs) (23, 25, 220), respectively. GPCRs are composed of seven hydrophobic, helical, membrane-spanning domains and a cytosolic G-protein binding site. G-proteins are heterotrimeric and consist of α, β, and γ subunits (357, 414). Each α-subunit has intrinsic GTPase activity at the guanine nucleotide binding site, and specific binding sites for effector proteins such as phospholipase C (PLC), adenylate cyclase, or other hydrolases. Once a ligand binds to its specific GPCR, guanosine diphosphate (GDP) is rapidly exchanged for GTP, which results in G-protein dissociation from the receptor and α-subunit dissociation from the β-γ complex. The α and β-γ complex each may then activate effector proteins in different biochemical pathways. When GTP is hydrolyzed by GTPase, the heterotrimeric G-protein complex reassociates and can be reactivated (188, 357). Several GPCRs are expressed and have been particularly well studied in the liver. These include the V1a vasopressin receptor, the α1B adrenergic receptor, several subtypes of the P2Y class of purinergic receptors, and the angiotensin receptors (129, 337, 338, 381).
RTKs exhibit ligand-activated protein kinase activity and have only one membrane-spanning helix, connecting the extracellular ligand-binding domain to the cytoplasmic protein tyrosine kinase domain. Kinase activity is switched on by receptor dimerization and transphosphorylation of tyrosine residues within the kinase activation loop in the catalytic cytoplasmic domain. This in turn leads to the recruitment of a series of adaptor proteins containing an SH2 (Src homology domain 2), SH3 (Src homology domain 3), or PTB (phosphotyrosine binding) activating signaling pathways that generally regulate cell proliferation, migration, and survival. Protein phosphatases may terminate the signal by dephosphorylating tyrosine residues to disassemble the signaling complexes formed around the activated receptors (25, 220, 350). Several RTKs are expressed in the liver (40, 64, 170, 331, 363, 373) including the hepatocyte growth factor (HGF) receptor c-Met, the epidermal growth factor (EGF) receptor, and the insulin receptor, and their actions are implicated in glucose and lipid metabolism (254, 358), liver fibrosis (18), regeneration (389), and hepatocellular carcinogenesis (181, 383, 389).
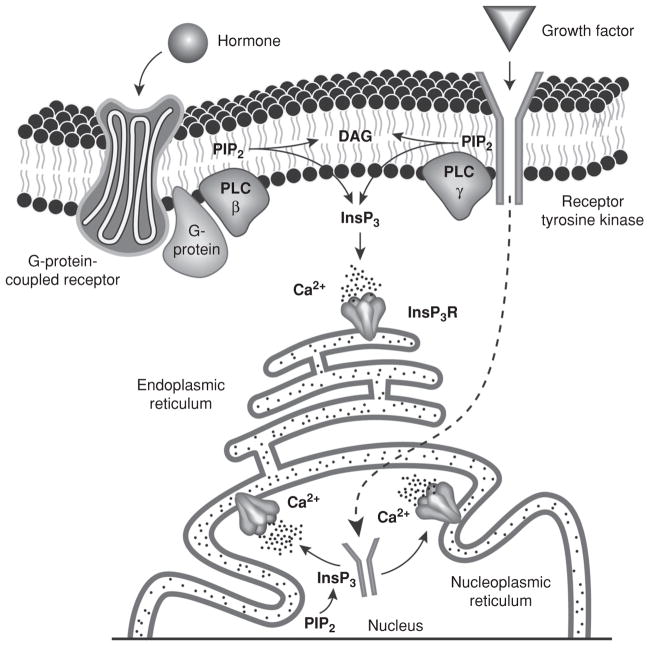
Once Ca2+-mobilizing hormones or growth factors bind to their specific plasma membrane receptors, membrane-associated PLC is activated. There are multiple subtypes of PLC (323), and several isoforms of these subtypes have been identified; in particular, PLCβ1 and PLCβ2 are activated by GPCRs, while PLCγ is activated by RTKs (25, 323). Activation of PLC by GPCRs hydrolyzes phospholipid phosphotidylinositol-4-5-bisphosphate (PIP2) in the plasma membrane, resulting in the formation of diacylglycerol (DAG) and InsP3. DAG remains at the plasma membrane to activate protein kinase C (PKC), while InsP3 diffuses into the cytosol to bind to the InsP3R, causing receptor opening and subsequent Ca2+ release from intracellular stores (25). Activation of PLC by RTKs was initially thought to similarly act on the plasma membrane pool of PIP2 (25) but evidence suggests it may alternatively hydrolyze nuclear PIP2 to generate InsP3 and Ca2+ release within the nucleoplasm (137, 331) (Fig. 1). The InsP3R is generally found in the membrane of the ER (367) and the nuclear envelope (NE) (176, 249) although InsP3Rs have been localized to the plasma membrane in certain tissues (92), as well as along the nucleoplasmic reticulum (NR) (see below) (176, 249).
Figure 1.
Molecular machinery for Ca2+ signal formation in hepatocytes. Ca2+ signals may be generated by Ca2+-mobilizing hormones (through activation of G-protein-coupled receptors) or growth factors (through receptor tyrosine kinases). Binding of a ligand to its specific receptor leads to the activation of phospholipase C (PLC), which hydrolyzes Phosphotidylinositol-4-5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (InsP3). DAG remains in the plasma membrane and InsP3 diffuses throughout the cytosol and binds to the InsP3 receptor (InsP3R) in the endoplasmic reticulum to allow Ca2+ release to the cytosol. Alternatively, receptor tyrosine kinases may translocate to the nucleus to locally activate PLC and induce Ca2+ release from the nucleoplasmic reticulum into the nucleoplasm. Nuclear PIP2 is depicted. However, its exact localization within the nucleus remains unknown [modified from references (211) and (137), with permission].
Three isoforms of the InsP3R have been identified and are denoted types I, II, and III (125, 240, 369). KO mice have been generated for each of the three isoforms (126, 248). InsP3R I deletion causes death of most of these mice in utero and the remaining have ataxia and tonic-clonic seizures. On the other hand, InsP3R II and InsP3R III double KO animals only show gross morphological or functional defects after postnatal day 20, when they manifest weight loss due to digestive system dysfunction (126). The InsP3R sequence contains approximately 2700 amino acids and its structure has six membrane-spanning domains with the large C-terminal end directed toward the cytoplasm and the narrower end facing the ER lumen (180). There are three regions within the InsP3R: an N-terminal InsP3-binding domain, a Ca2+ channel-forming C-terminal domain, and a regulatory domain flanked by the InsP3-binding domain and the channel region. Along their amino acid sequence, InsP3Rs contain several sites for phosphorylation by kinases such as cyclic AMP-dependent protein kinase (PKA), cyclic GMP-dependent kinase, PKC, Ca2+-calmodulin-dependent protein kinase II, and Akt kinase (191). Additionally, several protein partners interact with the regulatory domain to modulate the channel activity and with the C-terminal region to determine the subcellular localization of the receptor (32, 69). Upon InsP3 binding, the receptor undergoes a conformational change resulting in opening of the Ca2+ channel, to allow Ca2+ release from the ER into the cytosol. Each InsP3R isoform acts as an InsP3-gated Ca2+ channel (33, 151, 317). However, their sensitivity to InsP3 is not uniform, with type II being the most sensitive, followed by type I and lastly type III (277). Ca2+ release through the InsP3R requires InsP3 but the concentration of Ca2+ in the cytosol modulates the open probability of the Ca2+ channel (33, 150, 317). The open probability of Type I InsP3R exhibits a bell-shaped dependence on [Ca2+]i concentration. Therefore, incremental increases in [Ca2+]i concentration up to 1 to 2 μmol/L, sustain and amplify Ca2+ release, while higher concentrations become progressively inhibitory. This biphasic [Ca2+]i concentration dependence indicates that high concentrations of Ca2+ can feedback to turn the channel off, making the activity of the channel self-limiting (33). This property is responsible for Ca2+ oscillations (33, 88, 114). In contrast, the type III InsP3R exhibits a sigmoidal dependence on [Ca2+]i, and thus remains open in the presence of high Ca2+ concentrations. In this sense, activation of this isoform results in rapid and complete emptying of Ca2+ stores (150, 151). Single-channel studies of the type II InsP3R revealed that, like the type III lnsP3R, this isoform stays open in the presence of high Ca2+ concentrations (317). Unexpectedly, cells expressing only the type II isoform exhibit sustained [Ca2+]i oscillations, similar to what is observed in cells expressing only the type I InsP3R (261). This may reflect the fact that modifier proteins can alter the Ca2+ dependence of these isoforms (256).
The sequences of the InsP3R isoforms are considerably homologous but each subtype is expressed and regulated in a distinct fashion. There also are isoform-specific differences in tissue expression and subcellular distribution, suggesting that the various isoforms serve distinct roles in [Ca2+]i signaling (257). Particularly, hepatocytes express only type I and type II InsP3R, and type II is the major isoform (165, 409). The type III InsP3R is not detected in hepatocytes, but is the predominant isoform in the bile duct epithelial cells, cholangiocytes (163). Moreover, isoforms may be expressed in distinct sub-cellular locations. The InsP3R II is most concentrated in the apical region of hepatocytes, while InsP3R I is expressed throughout the cell (165, 267). In contrast, the InsP3R III is most concentrated in the apical region of cholangiocytes, while InsP3R I and II are dispersed throughout the cell (163). The apical localization of the type II and III InsP3R in hepatocytes and cholangiocytes, respectively, represents a specialized region for triggering polarized Ca2+ waves (see below) (163, 165).
Ca2+ can also be released from the ER through RyRs. These channels are activated by Ca2+ itself through a mechanism known as Ca2+-induced Ca2+ release (CICR), or by interaction with cADPR (25, 251, 253). Although RyRs seem to be absent from hepatocytes (135, 165), there is pharmacological evidence for RyR sensitivity, since RyR inhibition reduces the speed of hormone-induced [Ca2+]i waves (267). In addition, ryanodine binding sites have been identified in hepatic microsomes (356), and also a truncated RyR has been identified in hepatocytes, which increases the frequency of InsP3-induced oscillations, although it is not able to promote Ca2+ release (303). Moreover, there is evidence that CD38 (ADP-ribosyl cyclase), the enzyme that catalyzes the formation of cADPR from NAD+ is expressed in the liver (342). In rat hepatocytes, CD38 is mainly expressed in the NE, where it contributes to nuclear Ca2+ ([Ca2+]n) signaling (133, 192). Despite the different observations suggesting the presence of RyRs in the liver, their contribution to Ca2+ signaling in hepatocytes is an aspect that needs further investigation.
In addition to InsP3, Ca2+, and cADPR, NAADP can also promote Ca2+ release into the cytosol (25, 127). NAADP was originally found in sea urchin eggs (209) and is the most potent Ca2+ mobilizing messenger yet described (128). This intracellular messenger was initially shown to interact with lysosome-related acidic compartments (71, 418). Further evidence has revealed TPCs (7, 178) as the NAADP-activated Ca2+ release channels, with three differentially distributed isoforms (TPC 1, 2, and 3) (50, 58, 224, 340, 424). TPC2, in particular, has predominant lysosomal localization and has been identified in most human tissues, but with particularly abundant expression in the liver (58). NAADP interacts with high affinity with TPC2 (58), although recent evidence suggests that NAADP may bind to an accessory protein within a larger TPC complex (224). This interaction is tightly controlled by luminal pH and Ca2+ concentration (306). TPC2 mediates [Ca2+]i release after activation with low nanomolar concentrations of NAADP while micromolar concentrations desensitize the receptor. Moreover, NAADP-mediated Ca2+ release is subsequently amplified by CICR from InsP3Rs (58, 424). Therefore, this Ca2+ release from lysosomal stores may play an important role in shaping [Ca2+]c signals (60, 294). However, endogenous activators of NAADP and physiological functions of NAADP/TPC2-mediated Ca2+ release in the liver still remain elusive.
Mitochondrial Ca2+
In addition to the role that mitochondria play in the control of metabolism and respiration, these specialized organelles also contribute to the tight spatiotemporal control of Ca2+ signals by taking up Ca2+ from or releasing Ca2+ back to the cytosol (310, 343) (Fig. 2). In fact, mitochondria display an enormous capacity to accumulate Ca2+ in the very rapid time scale of hundreds of milliseconds (61) thus shaping and maintaining [Ca2+]c at resting levels.
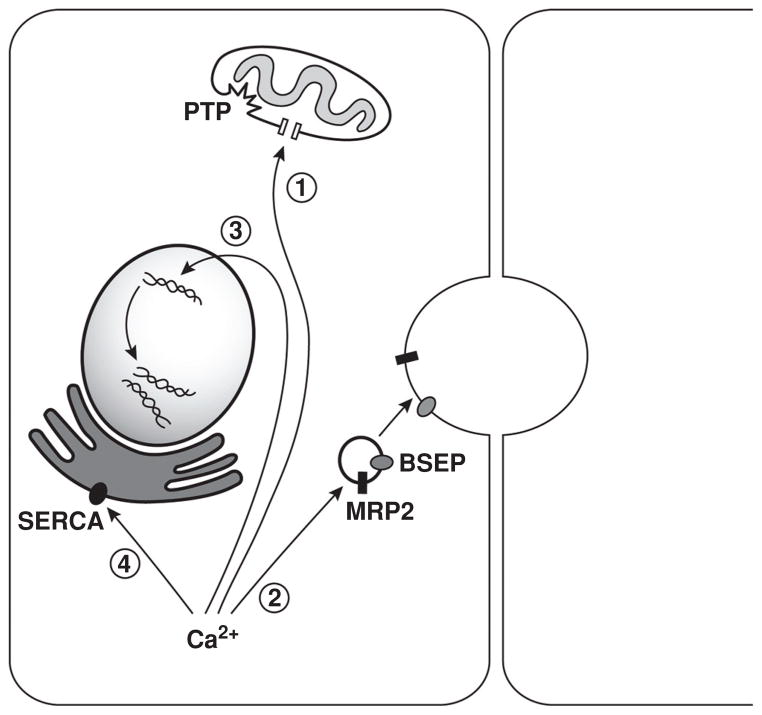
Figure 2.
Physiological and pathophysiological actions of Ca2+ in hepatocytes. 1. Cytosolic and mitochondrial Ca2+ signals are closely interrelated. Ca2+ is transmitted from inositol 1,4,5-trisphosphate receptors (InsP3Rs) to mitochondria, leading to an increase in mitochondrial free Ca2+ and the formation of the permeability transition pore (PTP). Reversible opening of the PTP is observed in normal mitochondrial function and shapes Ca2+ signals, but persistent opening of the PTP leads to leakage of cytochrome c, augmenting the cellular sensitivity to apoptotic stimuli. 2. Type II InsP3R-mediated Ca2+ release regulates organic anion and bile salt secretion through the insertion of the transporters multidrug resistance-associated protein 2 (MRP2) and bile salt export pump (BSEP), respectively, into the canalicular membrane. Loss of this isoform results in impaired transporter activity and contributes to the pathophysiology of intrahepatic cholestasis. 3. Nuclear Ca2+ is essential for cell-cycle progression through the control of gene transcription and expression of proliferative proteins. Furthermore, nuclear Ca2+ may be associated with liver tumor growth. 4. The endoplasmic reticulum (ER) regulates protein folding, quality control, trafficking, and targeting, and these depend on adequate amounts of Ca2+ in the ER lumen. Unbalance between ER load and protein-folding capacity leads to ER stress. In obesity, liver expression and activity of SERCA are impaired, leading to ER stress and defective glucose metabolism.
Ca2+ uptake across the inner mitochondrial membrane (IMM) is mediated by the ruthenium-red-sensitive [Ca2+]m uniporter (61, 81, 237, 349) and/or the rapid uptake mode, a transport mechanism that might contribute to [Ca2+]m uptake from fast [Ca2+]c transients in vivo (147). In addition, the outer mitochondrial membrane voltage-dependent anion channel may modulate Ca2+ access to the mitochondrial intermembrane space (136). This type of mitochondrial transport is driven by the potential gradient across the mitochondrial membrane (146). Ca2+ efflux occurs through the electrogenic Na+-Ca2+ antiporter and through a Na+-insensitive but H+-sensitive Ca2+ efflux pathway (22, 167). These efflux mechanisms are active transport systems (146). The molecular identity of these transport systems has remained largely elusive. However, recent studies have revealed novel candidate proteins that may mediate Ca2+ transport across the IMM (152). Ca2+ can also be released from mitochondria through the permeability transition pore (PTP), a voltage-dependent, high conductance channel that requires a permissive load of matrix Ca2+ for opening (20, 21, 80). Formation of the PTP results from increases in mitochondrial free Ca2+, membrane depolarization, and alkaline matrix pH (147, 299), and induces the PT, a permeability increase of the IMM to ions and solutes with molecular masses up to 1500 Da (20, 21, 80). Activation of the PTP leads to rapid release of large amounts of [Ca2+]m into the cytosol (173, 183). The PTP can be inhibited by antioxidants, reducing agents, and the immunosuppressive peptide cyclosporin A (21, 146, 278). Endogenous inhibitors of PT include adenosine diphosphate (ADP), Mg2+, and increased mitochondrial membrane potential (146). Persistent opening of the PTP can induce irreversible cell injury and death (20, 21, 80, 146, 276). On the other hand, reversible activation of the PTP is observed in normal mitochondrial function and plays a role in [Ca2+]i signaling (146, 173, 174) (Fig. 2). This is possible through several signaling steps; the mitochondrial membrane potential gradient allows [Ca2+]m uptake through the uniporter; this influx of cations is compensated by H+ efflux resulting in increased mitochondrial pH, which in turn activates formation of the PTP. This alters the membrane potential gradient, allowing Ca2+ to be released from the mitochondria, triggering [Ca2+]m-induced Ca2+ release (mCICR) (173). mCICR, in regions where mitochondria are densely distributed, allows Ca2+ waves to propagate across the cytosol (153, 173). Indeed, mitochondria participate in hepatocyte [Ca2+]i signaling (85, 153). This ability to contribute to the spatiotemporal control of Ca2+ signaling is due to the close association between mitochondria and [Ca2+]i release sites. This has been confirmed by the observation that [Ca2+]m can closely parallel the [Ca2+]i increase induced by receptor activation. Furthermore, the increase in [Ca2+]m following release of Ca2+ from intracellular stores is faster and larger than the increase that follows influx of extracellular Ca2+ (324). There is not only close proximity but also dynamic physical interaction between mitochondria and the ER (84, 348). Additionally, there is functional evidence for microdomains where mitochondria and InsP3Rs are in close apposition, so that mitochondria take up a significant fraction of the Ca2+ released by the InsP3R (85, 153, 183, 324). Approximately 30% of the total cellular mitochondrial pool is located in these microdomains of high [Ca2+]i (367). Of note, sub-cellular regions that contain fewer mitochondria show greater sensitivity to InsP3 (153). This property of mitochondria can locally modulate InsP3R sensitivity and thus define the threshold for InsP3 to trigger [Ca2+]i signals in different subcellular regions (153).
[Ca2+]m uptake affects multiple factors in cell metabolism such as mitochondrial proton motive force, electron transport and the activity of dehydrogenases associated with the tricarboxylic acid cycle (154, 326). In isolated hepatocytes, slow or small [Ca2+]i elevations are not transmitted effectively into mitochondria, and therefore, are unable to activate mitochondrial metabolism (326). Conversely, [Ca2+]i oscillations trigger [Ca2+]m oscillations and sustained nicotinamide adenine dinucleotide NAD(P)H formation (311). Therefore, the frequency rather than the amplitude of [Ca2+]i oscillations regulates mitochondrial metabolism (154, 311). Indeed, Ca2+ signaling in the cytosol and mitochondria are closely related, allowing the tight regulation of spatiotemporal Ca2+ signaling and cell metabolism.
Nuclear Ca2+
Most Ca2+ signaling machinery found in the cytoplasm is duplicated in the nucleus, and there is evidence that [Ca2+]n signals can occur independent of [Ca2+]c signals (106, 215). The nucleus is bordered by the NE, a specialized region of the ER, which has a double function as it insulates the nucleoplasm from the cytoplasm and also has the ability to store and release Ca2+ (207, 249, 279). It is noteworthy that the NE is not simply a smooth-surfaced outer boundary but is interrupted by invaginations that reach deep within the nucleoplasm and could even traverse the nucleus completely. The existence of such a complex branched network of invaginations forming a NR represents an additional regulatory Ca2+ domain within the nucleus (106, 234). Phosphoinositides (as well as the enzymes responsible for their synthesis, namely, phosphatidylinositol and phosphatidylinositol 4-phosphate kinases) have long been known to be present in the nucleus (176), although their precise localization within the nucleus remains unknown (177). Furthermore, different PLC isoforms have been identified in the nucleus and may provide in situ production of IP3 and DAG (74, 394). Both the NE and NR express InsP3Rs and numerous lines of evidence support a role for nuclear InsP3Rs in regulating [Ca2+]n release (57, 160, 171, 215, 238, 362). Of note, InsP3Rs can be found in the outer (ONM) (62, 233, 362) and inner nuclear (241, 249) membranes. Additionally, sarco/ER Ca2+ ATPase (SERCA) uptake pumps are found in the ONM and have been shown to be identical to those in the ER (207). Therefore, the nucleus expresses the machinery necessary for local Ca2+ release and reuptake. Such machinery may be activated selectively through tyrosine kinase pathways (73). Indeed, IGF-1 and integrins cause PIP2 breakdown in the nucleus but not at the plasma membrane (73), while activation of GPCRs causes breakdown of PIP2 in the plasma membrane, but not in the nucleus (307). Similarly, activation of the HGF receptor c-Met or the insulin receptor causes their rapid translocation to the nucleus, to selectively hydrolyze nuclear PIP2 and generate InsP3-dependent [Ca2+]n signals (137, 331). It also has been hypothesized that relocation of MAP kinase to the nucleus activates nuclear PLC to generate InsP3 in the nucleus (347).
[Ca2+]n signaling may also occur by passive transmission of [Ca2+]i signals into the nucleus through the nuclear pore complexes (NPCs), which are involved in transport of macromolecules from the cytoplasm to nucleoplasm and vice versa (366). In fact, this mechanism was originally thought to be the principal one responsible for formation of [Ca2+]n signals. The ion conductance of NPC is very large and this should allow free solute and ion diffusion. Then, [Ca2+]c signals should generate identical [Ca2+]n signals (3). It has been reported, however, that the NPC conductance can be dramatically reduced by accumulation of Ca2+ inside nuclear cisterna (gating) or by transport of macromolecules through the NPC (plugging) (365), although this view is controversial (132). Opinions on the Ca2+ permeability of NPC are also far from uniform. Both reports of free (51, 355) and restricted (11, 134, 230) permeability of Ca2+ through NPC have been shown. Studies monitoring diffusion of photoactivatable GFP across the NE suggest that permeability of the nuclear pore may be regulated by [Ca2+]i rather than by Ca2+ stores within the NE (285). In either case, since EF-hand Ca2+-binding motifs are present in proteins of the nuclear pore, it is possible that these function as Ca2+ gating sensors for the pore (347).
Although [Ca2+]n signals can originate inside the nucleus, and the permeability of nuclear pores to Ca2+ is regulated, there is considerable evidence that [Ca2+]n can passively follow [Ca2+]i (160, 225). For example, stimulation of hepatocytes with vasopressin results in a Ca2+ wave that appears to spread from the cytosol into the nucleus (223). Moreover, a mathematical analysis of Ca2+ waves in hepatocytes stimulated with vasopressin suggests that [Ca2+]n signals can be described simply by diffusion of Ca2+ inward from the NE (118).
[Ca2+]n can also contribute to Ca2+ signals in the cytosol. For example, Ca2+ puffs, which are highly transient and localized [Ca2+]i signals that result from the coordinated opening of small clusters of InsP3Rs, can spread across the cell by diffusing across the nucleus (421). Puffs can be triggered by a subthreshold concentration of agonist and the resulting [Ca2+]i signal rapidly dissipates by diffusion in the cytosol and sequestration of Ca2+ into intracellular stores. However, since the range of diffusion of Ca2+ in the nucleus can be much greater than in the cytosol (118), Ca2+ puffs generated near the NE can spread into and across the nucleus to spread to other, more distant regions of the cytosol (225). Thus, the nucleus may function as a path that helps distribute Ca2+ to the cytosol.
Ca2+ signaling patterns in individual cells
Ca2+ signals are highly organized in both space and time, from the subcellular to the cellular to the whole tissue level, to ensure reliability and specificity (24, 72, 105, 381). In the liver, this organization is achieved in the form of single transient or sustained [Ca2+]i increases, Ca2+ oscillations and [Ca2+]i waves (105, 381). In hepatocytes, Ca2+ oscillations originate from the periodic opening of Ca2+ channels located in the membrane of the ER, following activation of the PLC/InsP3/InsP3R cascade, and their shape and duration depends on the concentration and the type of agonist in use (413). For example, populations of isolated hepatocytes respond to agonists such as vasopressin, phenylephrine, or angiotensin with regular biphasic increases in [Ca2+]i, composed by two main events. The first is a rapid peak and then fall in [Ca2+]i, which occurs over a period of seconds. This is the result of Ca2+ release from InsP3-sensitive stores and does not depend on extracellular Ca2+, since it can be observed in Ca2+-free medium (189, 337). The second event is the sustained plateau in [Ca2+]i, which follows the rapid peak. This occurs only in the presence of extracellular Ca2+ that is necessary to reload depleted intracellular stores (155). In the case of vasopressin, lower concentrations induce [Ca2+]i oscillations, while higher concentrations induce sustained increases in [Ca2+]i. In contrast, stimulation of hepatocytes with phenylephrine or the bile acid ursodeoxycholic acid (UDCA) typically evoke [Ca2+]i oscillations at all concentrations (30, 337). Additionally, ATP stimulation leads to complex oscillations corresponding to a large peak, followed by smaller amplitude oscillations superimposed on a plateau phase (98). In all cases, the information of the agonist-induced Ca2+ increase is encoded in the rate of Ca2+ events or the amplitude of the Ca2+ rise, a phenomenon known as frequency encoding (26, 104, 105, 129). The duration of the [Ca2+]i oscillations can also be determined by the agonist (413). For example, phenylephrine induces shorter [Ca2+]i spikes than vasopressin or angiotensin (412, 413).
Frequency-encoded Ca2+ signals are thought to play an important role in determining the extent and targeting of [Ca2+]i signals to downstream Ca2+-sensitive proteins, yielding higher fidelity than other types of [Ca2+]i signals (e.g., amplitude modulated) (379, 381). In this sense, the frequency of [Ca2+]i spiking has been shown to differentially induce gene expression through the transcription factors, NFAT, NFKB, and OAP (100, 222, 384), to modulate Ras and the MAP kinase pathway (384) and to regulate mitochondrial metabolism (154) with greater efficacy than a single Ca2+ pulse or a persistent increase in [Ca2+]i. Decoding the information contained in a [Ca2+]i spike is essential to ensure the precise processing of Ca2+ signals. The activation and inactivation rates of Ca2+/calmodulin-dependent protein kinase II (87) and the Ca2+-dependent translocation of calmodulin (79), PKC (16, 304, 393) or the RAS GTPase activating protein, RASAL (397), suggests that these Ca2+-sensitive proteins can sense the frequency of Ca2+ signals and discretely modulate enzymatic activity. Mitochondria sense the frequency of oscillatory [Ca2+]i signals as well (325) by increasing the dehydrogenase NAD(P)H production in a time-averaged manner, thus modulating mitochondrial oxidative metabolism (326, 327).
Changes in InsP3 concentration are not the sole determinant of Ca2+ oscillations (246). Thapsigargin (117), which releases Ca2+ from the ER through an InsP3-independent mechanism, and nonhydrolyzable InsP3 analogs (396) can trigger [Ca2+]i oscillations. The bell-shaped dependence of the InsP3R open probability on [Ca2+]i concentration is thought to be one of the regulatory mechanisms responsible for oscillatory Ca2+ signals (33, 88, 317). Additionally, the maintenance of hormone-induced Ca2+ oscillations requires the constant replenishment of the ER with Ca2+ entering the cell through Ca2+ entry channels in the plasma membrane. Although several types of Ca2+ entry channels may be involved in maintaining proper Ca2+ concentrations in the ER, store-operated Ca2+ channels (SOCs), which are activated by a decrease in Ca2+ in the ER lumen, play a major role in liver cells (14, 15, 182). The liver expresses the SOCs Orai1 (a member of the Ca2+ release-activated channel modulator family of proteins) and transient receptor potential vanilloid 1 (TRPV1), which are plasma membrane Ca2+ channels, and the ER protein stromal interaction factor 1 (STIM1). It has been shown that these SOCs have a high selectivity for Ca2+ and that the Orai1 and STIM1 activation mechanism involves Ca2+ release from a putative small subregion of the ER likely close to the plasma membrane (15). However, the nature of such an ER subregion, its relationship with the bulk of the ER, and the steps involved in STIM1 interaction with Orai1 and other proteins still need to be determined in liver cells.
Ca2+ signals can also encode information spatially, in the form of [Ca2+]i waves (105, 381). These are characterized by an initial local increase in Ca2+ concentration, which then propagates in the whole cell at an approximately constant rate (104). This spatial organization has been observed in isolated hepatocytes (338, 381) and in individual cells in the perfused liver (328, 382). In nonpolarized preparations of hepatocytes, vasopressin, and phenylephrine induce [Ca2+]i waves that begin at a single locus, and this site of origin remains constant regardless of the agonist (338). The subcellular distribution of the InsP3R has been implicated in this spatial organization of Ca2+ waves (161). In polarized hepatocytes, the type II InsP3R (the most abundant isoform) is localized in the pericanalicular region (267) and this localized expression establishes a trigger zone from which Ca2+ waves originate (165) (Fig. 3). Additionally, the function of this trigger zone as an initiation site for Ca2+ waves depends on the integrity of lipid rafts (263). Similarly, the apical localization of InsP3Rs determines the initiation of Ca2+ signals in other cell systems (272, 417) including cholangiocytes, which express mostly the type III InsP3R (163). In these systems, InsP3 is generated by PLC activation near plasma membrane hormone receptors, which are often on the basolateral membrane, so it would be counterintuitive that Ca2+ signals instead would begin at the apical membrane. Certain biophysical properties of the type II (317) and III (150) InsP3Rs may explain why these receptors initiate Ca2+ waves at the trigger zone, but an alternative explanation is that clustering of InsP3Rs in the apical region lowers the threshold for Ca2+ release (371, 421). Indeed, the rise time is prolonged and the Ca2+ wave speed is reduced in hepatocytes with decreased expression or in which there is redistribution of the type II InsP3R (161).
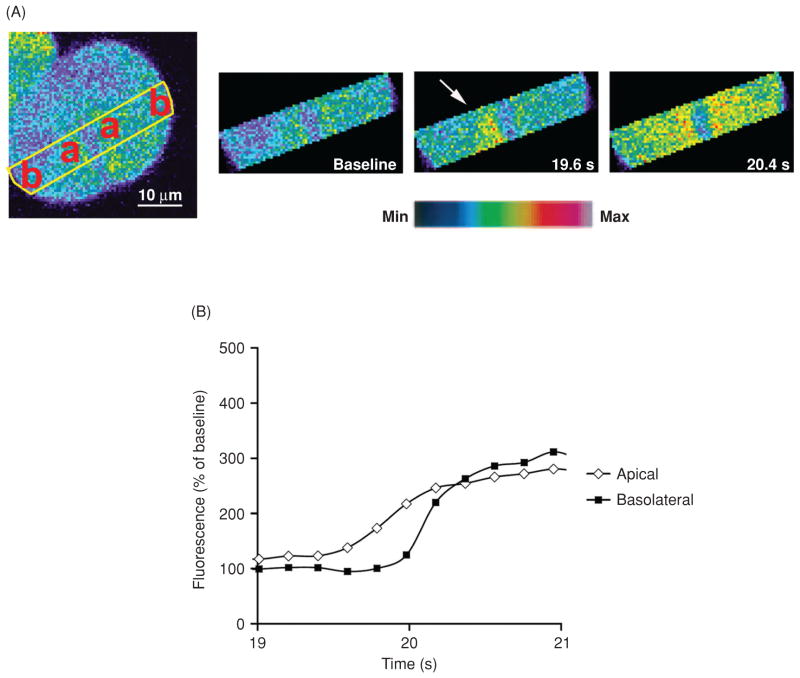
Figure 3.
Ca2+ waves begin in the apical region of hepatocytes. (A) Confocal images of an isolated rat hepatocyte couplet loaded with the Ca2+ sensitive dye Fluo-4/AM and stimulated with vasopressin. Serial images of the region of interest outlined in yellow show that a Ca2+ wave starts in the apical (a) region of the cell and spreads to the basolateral (b) region. Images were pseudocolored according to the scale shown at the bottom. (B) Graphical representation of the fluorescence increase in the apical and basolateral region shows that the apical Ca2+ signal precedes the basolateral Ca2+ signal [modified from reference (263), with permission].
The mechanism that governs apical-to-basolateral Ca2+ waves in hepatocytes remains elusive. In pancreatic acinar cells, the basolateral expression of the RyR Ca2+ release channel (213) may be the lead for Ca2+ wave propagation. RyR is activated by Ca2+ itself, which results in CICR. Therefore, InsP3 in the trigger zone initiates Ca2+ waves, which propagate across the cell by RyR-mediated CICR (172, 212). Cholangiocytes, on the other hand do not express the RyR but low levels of InsP3Rs are found in the basolateral region (163). Since Ca2+ can coactivate the InsP3R (33), Ca2+ waves in cholangiocytes likely propagate by a modified form of CICR in which Ca2+ released from the apical trigger zone sensitizes basolateral InsP3Rs allowing Ca2+ waves to form. In hepatocytes, pharmacological evidence using RyR inhibitors suggests that the spread of Ca2+ waves depends upon serial activation of apical InsP3Rs, and then basolateral RyRs (267), similar to what occurs in pancreatic acinar cells (212). However, RT-PCR studies suggest that hepatocytes lack RyRs (165). Conversely, a truncated but functional form of the RyR was detected in hepatocytes (303). The subcellular distribution of this novel RyR variant and its role in the formation of Ca2+ waves still needs to be determined. Of note, the speed or amplitude of [Ca2+]i waves in hepatocytes is not altered in Ca2+-free medium (267), which suggests that their propagation depends only on release of Ca2+ from intracellular stores. Furthermore, Ca2+ waves can be induced in hepatocytes by direct introduction of a nonmetabolizable analog of InsP3 (105) or by treating these cells with agents such as tert-butyl hydroperoxide or certain bile acids that raise Ca2+ in the cytosol independently of InsP3 (76, 336). These observations strongly suggest that Ca2+, and not InsP3, plays the major role in the propagation of the waves. However, a modeling system has suggested instead that InsP3, and not Ca2+, is necessary for InsP3-induced Ca2+ waves (179).
Intercellular communication: Spread of Ca2+ signals from cell to cell
[Ca2+]i waves are not confined to single cells, but rather a Ca2+ increase in one cell can be conveyed to neighboring cells giving rise to intercellular Ca2+ waves (129, 339). Ca2+ waves spread between hepatocytes (266), and this communication depends on gap junctions, which, in fact, are permeable to both Ca2+ and InsP3 (344). Either molecule could act, therefore, as the diffusible Ca2+-mobilizing second messenger (344). Additionally, hormone-induced [Ca2+]i signaling is highly coordinated in these cells, which depends upon gap junction conductance as well (266). Further evidence for a role of gap junctions has been provided by studies demonstrating blockage of intercellular Ca2+ waves by gap junction inhibitors (346, 385) or electroporation of antibodies to the gap junction components, connexins (38). Hepatocytes express two connexin isoforms, connexin 26 (Cx26), and connexin 32 (Cx32) (111), which is the most abundant in the liver (111, 204). Expression of both of these isoforms is dramatically reduced and coordination of [Ca2+]i signals is impaired after bile duct ligation (BDL) (111). Moreover, hepatocytes isolated from Cx32 KO mice have impaired intercellular propagation of Ca2+ signals (78, 282). Additionally, transmission of Ca2+ from cell to cell is possible by the expression of Cx32 or connexin 43 (Cx43) in a liver cell line where intercellular Ca2+ signals are not normally observed (214). Such findings show that gap junctions allow second-messenger communication to organize Ca2+ signals in adjacent cells. Furthermore, functional gap junction channels are essential for the spread of intralobular Ca2+ waves (129).
Other factors are required to support the spread of [Ca2+]i waves. Increases in InsP3 are necessary in each cell across which a Ca2+ wave spreads (39). Moreover, the presence of both InsP3 and Ca2+ is required to control the transmission of a [Ca2+]i wave across a hepatocyte (385). Additionally, Ca2+ waves only propagate if agonist is binding to its specific receptor at the surface of each cell. This likely means that the presence of hormone at each cell ensures that a sufficient level of excitability is achieved in the cytosol through the generation of the necessary levels of intracellular messengers, to support the propagation of a [Ca2+]i wave (328, 385). Intercellular Ca2+ waves are further organized by hepatocyte “pacemaker cells” (214). These cells have increased expression of hormone receptor, which makes them more sensitive to hormonal stimulation. Therefore, Ca2+ signals occur sooner in pacemaker cells than in other cells stimulated with the same concentration of hormone, due to higher InsP3 production that drives [Ca2+]i waves to neighboring cells (77). Of note, this increased sensitivity does not result from differences in downstream Ca2+ signaling components such as G-proteins or InsP3R (77, 387). This specialized organization might explain why vasopressin-induced waves begin in the region of the central venule, where the vasopressin V1a receptor is most heavily expressed, and then propagate to the portal region, where it is less heavily expressed (271). Additionally, pacemaker regions have been observed for other agonists like ATP and phenylephrine. Collectively, Ca2+ wave organization and propagation depends on several key aspects: InsP3 generation in each cell; a subset of cells with relatively increased expression of hormone receptor to initiate Ca2+ signals and thus serve as pacemakers for their neighbors; and gap junctions to coordinate the progression of Ca2+ signals among cells (56, 214).
Ca2+ waves may also be communicated between cells by paracrine signaling, involving the release of a messenger, its diffusion in the extracellular space, binding of the substance to a receptor, and activation of a signaling cascade that ultimately increases [Ca2+]c (104). One such extracellular messenger in the liver is ATP. This agonist is secreted by both hepatocytes (351) and bile duct cells (115, 259) and stimulates the G-protein-coupled P2Y receptors that are also expressed in both cell types (196, 250, 269). Therefore, secretion of ATP by hepatocytes stimulates InsP3-mediated Ca2+ signaling in neighboring hepatocytes, as well as in bile duct cells (351), creating a paracrine signaling system in which signal propagation to adjacent cells does not depend on intercellular communication through gap junctions.
Ca2+ signals also regulate specific functions in other liver cell types. For example, [Ca2+]i mobilization is implicated in the differentiation (activation) of hepatic stellate cells (HSCs) and portal (myo)fibroblasts during liver fibrosis (198, 292). HSC and portal fibroblasts express receptors for several Ca2+-mobilizing agonists, such as endothelin (198, 322), platelet-derived growth factor (PDGF) (86, 110), vasopressin (305) and ATP (102, 316), and upregulation of serotonin (5-HT2) receptors may be observed after HSC activation (292). Liver myofibroblast activation may exhibit different profiles of Ca2+ responses to endothelin and PDGF (198). Furthermore, CD38-mediated Ca2+ signals (through production of cADPR and NAADP) contribute to liver fibrosis via HSC activation (194). Moreover, activated fibroblasts and HSC are contractile due to their expression of motor proteins that can be regulated by Ca2+-dependent and Ca2+-sensitization mechanism pathways (175, 330, 420). In addition, InsP3R type 1 is shifted to the nucleus and to cell extensions upon HSC activation and Ca2+ release in these extensions promotes localized contractions (201).
Physiological Functions of Ca2+ Signals
Bile secretion
Bile secretion depends on the function of a number of membrane transport systems in hepatocytes and bile-duct epithelial cells (cholangiocytes) and on the structural and functional integrity of the bile-secretory apparatus (265). Ca2+ has been implicated in the regulation of such functions (Fig. 2). Agents that increase [Ca2+]c from either internal or external sources in isolated hepatocytes inhibit bile secretion in isolated perfused livers, independently of PKC or hemodynamic effects. Furthermore, inhibition of Ca2+ signals prevents this inhibition of bile secretion (273). These findings suggest that increased [Ca2+]c may play an inhibitory role in the regulation of hepatocyte bile production (273).
Possibly, Ca2+ inhibits bile flow by increasing paracellular permeability, which has been reported in isolated perfused livers and isolated hepatocytes (229), and would allow reflux of biliary constituents into the sinusoidal space, thereby dissipating the osmotic gradient that drives bile flow (274). In contrast to the inhibitory effect on net bile secretion, Ca2+ has been shown to mediate organic anion secretion through the insertion of the transporter Multidrug resistance-associated protein 2 (MRP2) into the canalicular membrane (83). Additionally, Ca2+ plays an important role in maintaining bile salt secretion through posttranslational regulation of the bile salt export pump (BSEP) (202) (Fig. 2). These effects are associated with pericanalicular Ca2+ release through the type II InsP3Rs that localize to this region in hepatocytes (165). These roles of Ca2+ in canalicular secretion can be supported by the observations that subplasmalemmal Ca2+ signals generally are obligatory for exocytosis (370), and Ca2+ acts as a regulator of exocytosis in the liver, in particular (53), suggesting that Ca2+ would be necessary for canalicular MRP2 and BSEP insertion. Moreover, the pericanalicular clustering of type II InsP3Rs creates a “trigger zone” that produces apical-to-basolateral Ca2+ waves in hepatocytes (165) which might promote bile secretion in a similar fashion to what has been observed in other polarized epithelia, including pancreatic acinar cells (187, 275) and cholangiocytes (116). These apparent discrepancies in the effects of Ca2+ on bile secretion in hepatocytes may be explained by differences in the nature of choleretic and cholestatic Ca2+ signals. Therefore, it may be important to consider that the magnitude, timing, and subcellular localization of Ca2+ signals all contribute to their physiological effects (72).
Canalicular contraction is a crucial step in conducting bile flow through the canalicular network and into the bile ducts (290, 399). Ca2+ mediates this process, since microinjection of Ca2+, or stimulation with vasopressin (266, 400) or ATP (398) promotes canalicular contraction in hepatocytes. Furthermore, [Ca2+]i increases result in the phosphorylation of myosin light-chain kinase which ultimately generates contractions in the pericanalicular actin network (415), which are organized within the hepatic lobule to form peristaltic waves (399). These waves are in turn essential to maintain bile flow (301).
The biliary tree plays an important role in conditioning the canalicular bile that is secreted by hepatocytes (265). Cholangiocytes perform this function through the regulation of biliary bicarbonate secretion (164). The prototypical and most extensively characterized pathway for bicarbonate secretion is initiated by stimulation of the secretin receptor which leads to formation of cAMP and activation of the cystic fibrosis transmembrane conductance regulator (CFTR). Chloride released into the ductular lumen then is thought to be exchanged for bicarbonate via a chloride-bicarbonate exchanger (186, 208). An alternative or secondary secretory pathway is stimulated by neurotransmitters such as acetylcholine (ACh) and autocrine agents such as ATP, and is mediated by InsP3 and [Ca2+]c (269). The downstream effect of Ca2+ is to activate Ca2+-dependent chloride channels on the apical plasma membrane, which in turn release chloride that is exchanged for bicarbonate via a chloride-bicarbonate exchanger (186, 208). Although these two pathways were initially thought to act separately, a cross-talk has been suggested by the observation that Ca2+ potentiates adenylyl cyclase activity and cAMP formation in cholangiocytes via a calcineurin-dependent pathway (6), which may represent an indirect mechanism for Ca2+-stimulated secretion. However, further evidence has suggested that cAMP- and CFTR-mediated secretion may have a more direct association with Ca2+ (259). Increases in cAMP result in apical, CFTR-dependent secretion of ATP, leading to stimulation of apical P2Y nucleotide receptors and subsequent activation of apical, type III InsP3Rs. This in turn activates Ca2+-dependent chloride channels and then bicarbonate secretion (101, 259). These observations suggest that cAMP acts through an autocrine loop that involves ATP release, activation of P2Y receptors, and then an increase in [Ca2+]c. Moreover, this might represent a convergence of secretory pathways in the cholangiocyte at the level of InsP3R-mediated Ca2+ release (259). Therefore, stimulation of basolateral M3 muscarinic receptors by ACh leads to type I and II InsP3R-mediated Ca2+ release. This Ca2+ in turn drives apical Ca2+-dependent chloride channels, resulting in biliary bicarbonate secretion. In contrast, stimulation of basolateral secretin receptors results in cAMP formation, subsequent activation of CFTR, and ATP-mediated bicarbonate secretion. Further studies revealed that this purinergic-signaling axis is present in both small and large cholangiocytes. ATP released from “upstream” small cholangiocytes lining the small intrahepatic bile ducts can be secreted into the bile, and can act as a paracrine signal to the large cholangiocytes lining the larger “downstream” bile ducts, stimulating Ca2+-dependent secretion. Moreover, small cholangiocytes, which do not express CFTR (120), respond to extracellular nucleotides through Ca2+-activated chloride efflux, representing an additional driving force for cholangiocyte secretion (411).
Primary cilia, which are sensory organelles expressed in the apical membrane of cholangiocytes, also relate to Ca2+ signaling and secretion. Primary cilia sense increases in osmolarity within the bile duct lumen, through the activation of the Ca2+-permeable ion channel TRPV4. This allows entry of extracellular Ca2+ into the cytoplasm, increases in bile flow and ATP release and bicarbonate secretion (141). Primary cilia can also sense and transduce mechanical stimuli from within the bile duct lumen, so that increases in bile flow activate both cAMP production and Ca2+ release (245).
Glucose metabolism
Glucose metabolism in the liver is controlled through the coordination of glycogen synthesis and breakdown. Ca2+ plays an important role in these events, modulating the phosphorylation and dephosphorylation, and hence the activity of the regulatory intracellular enzymes glycogen synthase and glycogen phosphorylase (35, 109, 200). Several Ca2+-mobilizing hormones have been implicated in these events. For example, vasopressin, angiotensin-II and α-adrenergic agonists stimulate PLC, InsP3 increase, and Ca2+ release from the ER, leading to the phosphorylation of glycogen phosphorylase, decrease in glycogen synthase activity, and glycogenolysis (107, 297). Nucleotides promote glycogenolysis in a similar fashion through the activation of P2Y nucleotide receptors in both rat and human hepatocytes (96, 97, 190). The bile acids UDCA, lithocholic acid, and taurolithocholic acid (TLCA) activate phosphorylase in a comparable way to vasopressin (48, 75), although through a Ca2+-dependent but InsP3-independent manner (48). Physiological levels of glucagon can evoke Ca2+ increases in the liver (67, 361) by activation of either InsP3-dependent or cAMP-dependent signaling pathways, resulting in glycogen phosphorylase activation (361, 395). The glycogenolytic capacity of the liver is regulated by several factors such as the distribution of gluconeogenic enzymes, which are localized in the periportal region (184). Perhaps for this reason, ATP and glucagon stimulate glucose release mostly in the periportal area. On the other hand, vasopressin and norepinephrine stimulate glucose release in pericentral hepatocytes, which can be explained by the fact that these cells are more sensitive to such agonists than periportal hepatocytes (129, 386). This differential sensitivity to glucose-mobilizing hormones is overcome by intercellular communication, which coordinates the regulation of glucose metabolism across the whole liver through gap junctions. For example, norepinephrine or glucagon-induced glucose release is impaired in isolated perfused livers from Cx32-deficient mice (368). In an analogous way, treatment of isolated perfused livers or isolated rat hepatocyte couplets (107) with a gap junction blocker decreases vasopressin or glucagon-induced glucose release. However, gap junction inhibition does not affect glycogen breakdown induced in a receptor-independent fashion. Moreover, hormone-induced glucose release is also impaired if hepatocytes are dispersed (107). Collectively, these observations demonstrate that gap junctions play a very important role in the control of hormone-induced glucose release in the liver in response to the heterogeneous distribution of hormone receptors. Stress conditions can challenge this coordination of metabolic function; Cx32-deficient mice become hypoglycemic in response to fasting, whereas WT animals do not, and endotoxin-induced hypoglycemia is aggravated in Cx32-deficient mice (78). Because the coordination of glucose storage and release assures balanced glucose metabolism in the liver, the actions of these two pathways are complemented by one another to maintain homeostasis. For example, insulin antagonizes the glycogenolytic activity of glucagon and α1-adrenergic agonists in the liver (34, 89, 407). It is thought that insulin interferes with glucagon by reducing elevated levels of cAMP (34, 159, 407), whereas insulin inhibits the effects of α1-agonists by interfering with their ability to elevate cytosolic free Ca2+ (380).
Cell proliferation
Ca2+ plays an important role in cell proliferation (143, 364) as well and in progression of the cell cycle (185, 308, 318, 334, 390). Temporal and spatial patterning of Ca2+ signals determines their specificity, and Ca2+ signaling patterns can vary in different parts of the cell (28, 72, 157, 215). For example, oscillation frequency, amplitude, and time delay of cytosolic calcium oscillations are essential kinetic parameters to determine entry into the cell cycle (403, 404). Furthermore, the proliferative ability of calcium is closely related to the subcellular compartments where it is released. The heterologous expression of the Ca2+-binding protein parvalbumin (PV) has been used to study the role of Ca2+ signaling in the regulation of the cell cycle. PV variants targeted to the nucleus or the cytoplasm were used to investigate the relative importance of Ca2+ signals in each of these cellular compartments for regulation of the cell cycle in the hepatoma cell lines SkHep1 and HepG2. It was found that nucleoplasmic rather than [Ca2+]c is essential for cellular proliferation, and is necessary, in particular, for progression through early prophase (332) (Fig. 2). On the other hand, both cytosolic and nuclear Ca2+ signals were important for proliferation of LX-2 immortalized human HSCs and primary rat HSCs through CaMK II-mediated regulation of Cdc25C phosphorylation (359). Additional studies led to the observation that receptors for HGF, EGF, and insulin, three potent growth factors in the liver, translocate to the nucleus to generate InsP3-dependent Ca2+ signals (9, 137, 331), suggesting that certain growth factors may stimulate proliferation of hepatocytes by selectively inducing Ca2+ signals in the nucleus.
What are the specific targets of Ca2+ signals in the nucleus that are responsible for cell proliferation? [Ca2+]n signals activate the transcription factors cAMP response element binding (CREB) (157) and Elk1 (314) and stimulate the intranuclear activity of PKC (106) and CaMK-IV (90), which would be expected to stimulate cell proliferation (Fig. 2). A recent study used a rapid subtraction hybridization (RaSH) approach to select genes whose expression was affected by a small alteration in [Ca2+]n concentration. Legumain (LGMN), an asparaginyl endopeptidase involved in tumor metastasis was identified in this screen as a novel specific target for [Ca2+]n signals in SkHep1 cells. [Ca2+]n buffering resulted in decreased LGMN expression. Furthermore, LGMN gene knockdown resulted in decreased cell proliferation, suggesting that expression of this protein is associated with increased [Ca2+]n-dependent proliferation. These findings highlight a new role for LGMN and provide evidence that [Ca2+]n signals regulate cell proliferation in part through the modulation of LGMN expression (8).
Ca2+ also plays an important role in the proliferation of bile duct cells. The proliferative activity of large cholangiocytes is regulated by the activation of cAMP-dependent mechanisms (4, 121, 219). On the other hand, small cholangiocyte proliferation is regulated by the activation of α1-adrenergic receptors and occurs through Ca2+/calcineurin-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Furthermore, histamine stimulates small cholangiocyte proliferation through the activation of the H1HR receptor and InsP3/Ca2+ signaling (120, 122). These findings implicate α1-adrenergic and histamine receptors (in particular, H1HR) as key factors in the regulation of normal cholangiocyte proliferation and function. This is also important in pathological ductopenic conditions associated with damage of large ducts in which Ca2+-dependent small cholangiocyte proliferation may be a key compensatory mechanism for maintaining homeostasis and overall bile duct function (219, 239).
Apoptosis
Apoptosis can result from a variety of extracellular or intracellular stimuli, which converge with activation of caspases to lead directly to cell death (227). Apoptosis generally occurs through one of two pathways. The extrinsic pathway is initiated by death receptors of the tumor necrosis factor receptor superfamily (108). The intrinsic pathway involves the convergence of intracellular stress signals on mitochondria, resulting in the formation of the PTP (10, 63). In many cells, the death receptor pathway may act through mitochondria as well by inducing the cleavage of the proapoptotic molecule Bid and subsequent mitochondrial permeabilization (231). Therefore, mitochondrial permeabilization is key to both pathways. Apoptosis is modulated by a number of proteins in part through the InsP3R or InsP3R-induced Ca2+ release mechanisms (Fig. 2). These in turn mediate effects in [Ca2+]m, since a subset of mitochondria are in close proximity to InsP3Rs (324), and mitochondrial and [Ca2+]c signals are interrelated (154, 183). In particular, the mitochondrial apoptotic pathway is inhibited by members of the Bcl-2 family of proteins, including Bcl-2, Bcl-xL, and Mcl-1 (1, 144, 243, 321). Bcl-2 inhibits apoptosis in part by decreasing the size of ER Ca2+ stores (17), whereas Bcl-xL acts in part by inhibiting expression of the InsP3R (221, 405). Collectively, this reduces Ca2+ release from the ER into the cytosol and consequently decreases the amount of Ca2+ transmitted to surrounding mitochondria. Since mitochondria are less likely to become overloaded with Ca2+, the formation of the PTP is prevented and the overall result is a diminished cellular sensitivity to apoptotic stimuli (17, 221) (Fig. 2). In fact, overexpression of Bcl-xL provides protection against apoptotic cell death in rat hepatoma cells (124). On the other hand, neither InsP3R expression nor ER Ca2+ stores are affected by Mcl-1. Rather, Mcl-1 inhibits Ca2+ signaling directly within mitochondria (258). In contrast, the proapoptotic Bcl-2 family members Bax and Bak promote ER Ca2+ overload through the control of InsP3R phosphorylation (287), which leads to sensitization of mitochondria to calcium-mediated fluxes (284). These alterations serve as important upstream signals for cytochrome c release (283) (Fig. 2). Additionally, cytochrome c that has leaked from mitochondria via the PTP binds to the InsP3R, which facilitates release of toxic amounts of Ca2+ from the ER (36). This is thought to result in a positive feedback loop that causes further Ca2+ overload of mitochondria and then further leakage of cytochrome c (36). Inhibition of the interaction between cytochrome c and the InsP3R inhibits development of apoptosis by blocking this positive feedback loop (37). Therefore, Bcl-2 family members regulate their pro- or antiapoptotic functions at least in part through a range of complementary effects on Ca2+ signaling pathways.
Mitochondrial permeabilization and cell death can also be induced by dysregulation of redox transition, namely, the uncontrolled generation of reactive oxygen species (ROS) (216, 298). Oxidants increase [Ca2+]i load by stimulating IP3Rs in the ER, while inhibiting SERCA pumps and Ca2+ extrusion via plasma membrane channels (59). In turn, [Ca2+]m uptake stimulates oxidative metabolism, resulting in enhanced ATP production, and hence ROS generation as a byproduct of oxidative phosphorylation (320). Above a certain degree of [Ca2+]m and ROS rise, a progressive Ca2+ surge prompts a feed-forward loop that induces prolonged PTP opening and commits cells to death (298) by the combination of a set of events: IMM depolarization, which causes cessation of oxidative phosphorylation and ROS production; matrix swelling and cristae unfolding, and the ensuing breaches in the OMM with release of stored Ca2+ and of apoptogenic proteins (20, 80, 142, 319). In fact, Ca2+-induced production of ROS has been shown to promote cytochrome c release in rat liver mitochondria through mitochondrial permeability transition-dependent mechanisms (300). Furthermore, an analogue of the lipid peroxides formed during oxidative stress, tertbutyl-hydroperoxide, prompts NAD(P)H oxidation, increasing mitochondrial free Ca2+ and ROS, eventually leading to PTP opening and cell death (216).
It is important to note that when PTP induction is transient or limited to a fraction of mitochondria in a cell, a short burst of ROS production could occur. These ROS could serve as signaling components to propagate waves of short PTP openings in the surrounding mitochondria, producing additional ROS with possible functions in the regulation of chaperones, kinases, and gene expression. Hence, PTP could use Ca2+-dependent and independent ROS generation to tune many cellular routines unrelated to death induction (195).
Ca2+ Signals in Liver in Health and Disease
Liver growth: Regeneration versus hepatocellular carcinoma
The role of Ca2+ in cell proliferation assumes special importance in the process of liver regeneration. Liver regeneration after the loss of hepatic tissue is a fundamental parameter of the response to liver injury (255). It is induced by acute damage to the organ and is regulated in a well-orchestrated manner by cytokines, growth factors, hormones, and neurotransmitters that help promote massive proliferation of hepatocytes, which switch from a quiescent to a proliferative phenotype. This results in the restoration of liver mass and function within days after partial tissue loss (112, 377). A number of Ca2+ mobilizing hormones and growth factors are involved in this process of proliferative signaling. These include adenosine triphosphate (ATP) (138), arginine vasopressin (AVP) (281), noradrenaline (82), HGF (12, 260), EGF (260, 373), and insulin (331).
Alterations in components of the Ca2+ signaling machinery, namely, the different InsP3R isoforms and SERCA pumps, have been reported to occur during liver regeneration (95, 113, 232). Other reports have suggested that the sensitivity of liver cells to Ca2+ mobilizing agonists (169), as well as agonist-induced calcium signals are modified after partial hepatectomy (PH) in the rat (197). Another study suggested that these modifications reflect the remodeling of Ca2+ signals, which is thought to be essential for the initial regenerative response (280). Thus, Ca2+ signals are thought to adapt to the context of regulated proliferation induced by PH. Is there a fine regulation mechanism for proliferation during liver regeneration and does Ca2+ signal compartmentalization influence this process? Lagoudakis et al. investigated the role of [Ca2+]c during liver regeneration in the rat, targeting PV to this compartment (206). The study demonstrated in vivo, at the organ level that hepatocytes require unaltered cytosolic calcium signaling to progress through the cell cycle after PH, even though liver cell lines do not share this requirement, as discussed above (332). This may reflect the fact that primary hepatocytes are more sensitive than liver cell lines to Ca2+ buffering, since buffering of Ca2+ in the nucleus of primary hepatocytes in vivo leads to widespread apoptosis, liver failure, and death (unpublished observation). Alternatively, perhaps this is due to the fact that primary hepatocytes and liver cell lines do not express the same repertoire of intracellular calcium channels in the different cell compartments (215). Progression through the cell cycle from G0 to G1 and S phases is triggered presumably by Ca2+ mobilizing agonists that are known to be released in the early stages of liver regeneration after PH, such as HGF and EGF (260), ATP (138), AVP (281), and noradrenaline (82). Furthermore, [Ca2+]c signals may be important for other early events after PH, such as gene transcription and phosphorylation of ERK and CREB, which are crucial for hepatocyte progression after PH (341, 352, 372), and which are dependent on cytosolic and/or nuclear Ca2+ signaling (91, 99, 157, 205). Specific targets of [Ca2+]c signals during liver regeneration remain unknown.
A recent report has shown that [Ca2+]m signals also play an important role in the progression of liver regeneration after PH (145). Experiments in SkHep1 cells expressing PV in the mitochondria revealed that buffering of [Ca2+]m leads to decreased apoptosis through altered expression of proapoptotic and antiapoptotic proteins. In vivo studies showed that [Ca2+]m buffering accelerates liver regeneration after PH. Therefore, the regulation of apoptosis by [Ca2+]m signals is an additional mechanism by which hepatocyte proliferation and liver regeneration may be modulated. Future advances in this field should lead to a better understanding of how these various Ca2+ compartments act in an integrated manner to regulate liver regeneration.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy in adults and the third most common cause of cancer death worldwide (228). Its complex molecular pathogenesis is regulated by several signaling pathways (391, 392, 406). In particular, the role of growth factors, which act in part through [Ca2+]i release (28), has been highlighted in various studies and their signaling pathways are known targets for the discovery of new therapeutic approaches (391, 392, 406). In particular, HGF and its receptor c-met have been implicated in tumor invasion in HCC (247). The versatility of MET-mediated biological responses is modulated by subcellular compartmentalization of MET signaling pathways (389). In fact, c-met translocates to the nucleus upon HGF stimulation to generate InsP3-dependent Ca2+ signals there, and this process is thought to regulate cell proliferation (137). The invasive potential of HCC cells has also been linked to altered store-operated Ca2+ entry (148), through regulation by βig-h3, an extracellular matrix protein that is implicated in tumorigenesis and metastasis (374, 378). Selective buffering of nuclear but not [Ca2+]c also impairs growth of liver tumors in vivo (332) (Fig. 2). Additionally, [Ca2+]n signals mediate cell proliferation through the regulation of the asparaginyl endopeptidase LGMN (8). The observations that HGF stimulates increased expression of this protein, and expression also is increased in tumor cells compared to normal cells in liver specimens from patients with hepatocellular carcinona (HCC), suggests that LGMN’s positive effects in cell proliferation may promote carcinogenesis.
Two therapeutic agents, interferon-alpha (IFNα) and 5-fluorouracil (5-FU) are used experimentally in combination against HCC (264, 295, 345). A recent study revealed that [Ca2+]i might be a potent upstream proapoptotic messenger acting at the early stage of IFN-α/5-FU-induced apoptosis through the mitochondrial pathway (422). Furthermore, the novel squamosamide derivative and potent antioxidant N-(2-(4-hydroxy-phenyl)-ethyl]-2-(2,5-dimethoxy-phenyl)-3-(3-methoxy-4-hydroxy-phenyl)-acrylamide also known as FLZ, has been shown to have antitumor activity in HCC cells via modulation of the expression or activation of cell-cycle regulatory proteins, which are associated with decreased Ca2+/ROS levels. Therefore, FLZ represents a potential therapeutic agent for the treatment of HCC (315). Understanding the interplay between Ca2+ signaling and other signaling pathways, and how compartmentalized Ca2+ signals may control cell proliferation will reveal new clues of HCC pathogenesis, with the potential to lead to the discovery of more specific and effective therapeutic approaches.
Cholestasis
Bile secretion is one of the primary functions of the liver and defects in this process result in cholestasis. Chronic cholestatic conditions of the liver are among the most serious forms of liver disease. These conditions often result from diseases of the bile ducts (329, 388). Secretion in cholangiocytes can be mediated in part by Ca2+ (101, 164). More specifically, InsP3-mediated Ca2+ signals are important for normal bile secretion as discussed above, and loss of InsP3R is a common event in cholestasis (312). In particular, apical type III InsP3R expression is greatly decreased or absent in the bile ducts of patients with a range of cholestatic disorders including bile duct obstruction resulting from stone disease or malignancy, biliary atresia, primary biliary cirrhosis and sclerosing cholangitis (354). In accordance with these observations, animals subjected to common BDL or lipopolysaccharide (LPS) injection, which are accepted models of cholestasis (329, 354), had selective loss of InsP3R expression in biliary epithelia (354) (Fig. 4). As a result, Ca2+ signaling and Ca2+-mediated bicarbonate secretion is impaired. However, upstream components of the Ca2+ signaling pathway are preserved, which suggests that the selective decrease in InsPRs is the cause of defective Ca2+ signaling. Furthermore, this decrease in InsP3Rs is thought to interfere also with cAMP-mediated ductular secretion (218, 259). The loss of InsP3R from bile duct epithelia has been observed in other experimental animal models of cholestasis (5, 203, 217, 262, 360, 388). These findings may be explained by the fact that type III is the most abundant InsP3R isoform in cholangiocytes (163), and contribute to the formation of apical-to-basolateral Ca2+ waves (163, 166, 270, 409), which are critical for secretion in bile duct cells, due to their role in the transport of fluid and electrolytes across the apical membrane (116).
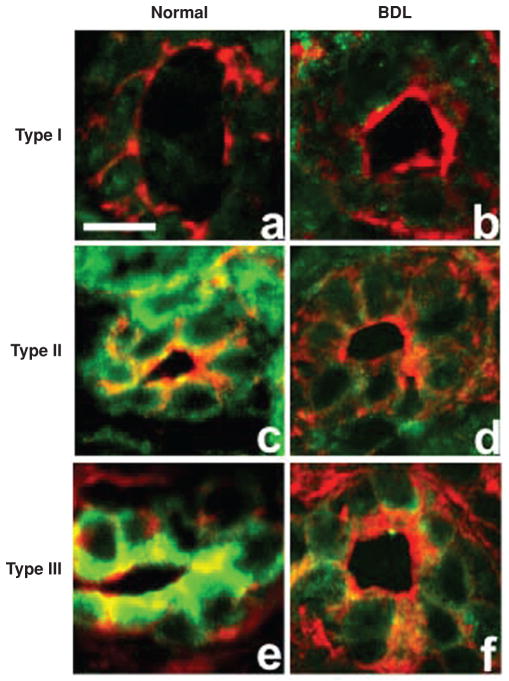
Figure 4.
Inositol 1,4,5-trisphosphate receptors (InsP3R) expression is lost in bile duct epithelia after bile duct ligation. Confocal immunofluorescence of liver sections from normal rats and rats subjected to bile duct ligation (BDL) labeled with isoform-specific InsP3R antibodies (green) and rhodamine phalloidin (red). Type 1 InsP3R labeling in normal bile duct cells (A) is found throughout each cell although is expressed at low levels, similar to that observed 2 weeks after BDL (B). Type 2 InsP3R labeling is seen throughout each cell in normal liver sections (C) and it is nearly absent 2 weeks after BDL (D). Type 3 InsP3R labeling is found predominantly in the apical region of bile duct cells in normal liver sections (E) and is also markedly reduced 2 weeks after BDL (F) [reprinted from reference (354), with permission].
Although cholestasis is often associated with defects in the bile ducts (329, 388), genetic or acquired disorders of hepatocyte transporters can also account for this condition (388). Recent studies have demonstrated that insertion of the transporter MRP2 in the plasma membrane is Ca2+ dependent and loss of type II InsP3R results in impaired canalicular organic anion secretion in hepatocytes (83) (Fig. 2). Moreover, loss of this isoform impairs the choleretic effect of tau-roursodeoxycholic acid (TUDCA) in cells treated with the cholestatic bile acid TLCA (83). Similarly, loss of type II InsP3R expression or loss of pericanalicular expression of the receptor leads to impaired BSEP-dependent secretion in hepatocytes (Fig. 2), and is observed in animal models of estrogen and endotoxin-induced cholestasis (202). Indeed, decreased type II InsP3R expression in the liver had been observed in an earlier study in animals subjected to BDL (103) and mistargeting of hepatocellular transporters is a common feature of cholestatic disorders (333). Being the most expressed isoform in this cell type, and localized preferentially to the canalicular region (267), the type II InsP3R regulates polarized Ca2+ waves (165), which in turn may regulate secretion in an analogous manner to what takes place is cholangiocytes (259, 354).
The mechanisms by which the decrease in InsP3R expression occurs remain unknown. Certain hepatic proteins can have decreased expression in BDL and other cholestatic conditions through the activation of nuclear hormone receptors like the farnesoid X receptor (FXR) (94). However, it is not known whether the promoter regions of any of the InsP3R isoforms contain response elements for FXR. Proinflammatory cytokines released during cholestasis are known to inhibit fluid secretion in cholangiocytes, so one could question their contribution to decreased expression of InsP3R. However, it has been shown that proinflammatory cytokines do not impair Ca2+-mediated secretion in these cells (360). Consistent with this observation, interleukin 1 increases rather than decreases transcription of the type I InsP3R gene (49). Perhaps increased degradation rather that decreased production could cause the observed loss of InsP3R expression. Indeed, InsP3R ubiquitination and proteasomal degradation are triggered upon secretagogue stimulation in pancreatic acinar cells (410).
These findings suggest that in canalicular cholestasis, decreased expression and localization of canalicular transporters results in part from loss of pericanalicular Ca2+ signaling. This has implications for the treatment of cholestatic disorders. UDCA, the only therapy of proven benefit for certain types of chronic cholestatic liver disease (296, 309), ameliorates liver function abnormalities in part by stimulating biliary exocytosis in a Ca2+-dependent fashion (30, 31). Moreover, these effects are partially mediated by the cooperative action of Ca2+-dependent conventional PKC alpha (cPKCα), which promotes transporter insertion into the canalicular membrane (29), and PKA (408), which phosphorylates the type II InsP3R potentiating InsP3-evoked Ca2+ release (52, 55). Furthermore, the cytoprotective effects of UDCA and its taurine conjugate TUDCA are associated with enhancement of Ca2+ signaling, inhibition of which blocks the activation of key survival pathways (244). In fact, UDCA stimulates secretion of the Ca2+-mobilizing agonist ATP from hepatocytes into bile, which may promote bile flow and act on downstream bile-duct epithelia by stimulating fluid and electrolyte secretion (268). Additionally, UDCA can locally stimulate cholangiocytes to release ATP and initiate P2Y receptor-mediated Ca2+-dependent fluid secretion (115). These findings are consistent with the hypothesis that stimulation of certain types of Ca2+ signaling in bile ducts and hepatocytes may provide a strategy for the treatment of cholestatic diseases. Taken together, loss of apical InsP3Rs in cholangiocytes and hepatocytes appear to be a common basis for impaired secretion and the development of cholestasis. These findings suggest a choleretic effect for Ca2+ through localized Ca2+ release in the pericanalicular region. In contrast, earlier studies had suggested a cholestatic effect, in which global Ca2+ signals induced by ionophores may promote cholestasis (273). Understanding the localized nature of these, Ca2+ signaling microdomains may provide insight into mechanisms of cholestasis and lead to the discovery of new alternatives to treat this condition.
The role of gap junctions in cholestasis has also been reported. Correa et al. investigated the involvement of Cx32, the major gap junction protein in the liver (111, 204), in the hepatic response to cholestasis (78). This study demonstrated that Cx32 deficiency impairs intercellular communication and the transmission of Ca2+ signals between hepatocytes after treatment with endotoxin. This finding is consistent with previous observations that Ca2+ waves and Ca2+ oscillations depend upon gap junctions in hepatocyte couplets (266), and that decrease in Cx32 protein levels after BDL is associated with impaired coordination of hormone-induced Ca2+ signals (111). Furthermore, Cx32 deficiency in mice leads to prolonged cholestasis when compared to wild type animals, suggesting that Cx32 expression is important for recovery from cholestasis after treatment with endotoxin (78). This report proposed an important role of gap junctions in the liver, to assure integrated and uniform tissue response in times of stress (78). Similarly, a subsequent study showed that cholestatic bile acids inhibit gap junction permeability and prevent the coordination of Ca2+ oscillations in hepatocytes and cholangiocytes (47). These findings suggest that this inhibitory effect might contribute to the cholestatic effect of these bile acids and may be an early event before the downregulation of Cx32 that has been observed previously in other models of cholestasis (111, 139). In contrast, opposing results were shown subsequently, in which TLCA-induced cholestasis was partially reversed by TUDCA in both wild type and Cx32-deficient mice, suggesting that gap junctional intercellular communication is neither essential for the cholestatic effect of TLCA nor needed for the choleretic effect of TUDCA (313). In aggregate, these observations are consistent with the idea that altered Ca2+ signaling is involved in the pathogenesis of cholestasis and can be modulated for therapeutic benefit, but careful examination of the response of patients to cholestasis may ultimately be necessary to establish a common basis for this disorder and the relevance of these observations for human disease.
Viral hepatitis
Viruses encode proteins and utilize host cellular proteins and signaling pathways to enhance viral replication, so regulating cellular Ca2+ signals is an ideal strategy for altering the intracellular environment to favor viral replication (66). Hepatitis B virus (HBV) is a para-retrovirus that affects more than 350 million people worldwide and chronic infection with HBV is the major cause for the development of HCC (158). The multifunctional HBV protein HBx is essential for replication (68, 425) and is thought to make significant contributions to the development of HBV-associated HCC (44). Elevation of [Ca2+]c signals as a result of HBx has been reported in several studies. HBx regulation of [Ca2+]c was shown to be essential for HBV replication in HepG2 cells and cultured primary rat hepatocytes (46, 130); calcium signals also promoted HBV capsid assembly in HepG2 cells (70). HBx elevation of [Ca2+]c signals is required for other activities of HBx, such as regulation of cell proliferation, activation of Pyk2/FAK-Src kinases, and activation of the transcription factor AP-1 (43, 45, 46, 131, 288). A very recent study added to these findings proposing that HBx stimulates HBV replication through increases in [Ca2+]m uptake, which promotes ER Ca2+ depletion and increased stored-operated Ca2+ entry, causing an increased plateau level of [Ca2+]c spikes (419). Indeed, continuing studies geared toward the discovery of the precise molecular basis of HBx regulation of Ca2+ signaling may help identify specific targets that regulate HBV replication and HCC development.
Hepatitis C Virus (HCV) also alters Ca2+ signals upon infection. HCV infection is a major cause of chronic hepatitis, liver cirrhosis, and HCC, affecting more than 170 million people worldwide (353). Several studies have suggested that HCV promotes alterations in mitochondrial oxidative metabolism (289, 376). Piccoli et al. provided insight into the mechanism by which this may occur, demonstrating that HCV protein expression results in Ca2+ overload in the mitochondrial compartment, which triggers alterations in the bioenergetic balance and nitro-oxidative stress (302). This study proposes that these effects are primed by an HCV protein pool localized on ER-associated mitochondrial membranes. In this model, HCV-induced ER stress, which has been documented previously (19, 375, 376), may provoke depletion of ER Ca2+, which in turn may be captured by the mitochondria to result in the described alterations (302). Cumulatively, these results suggest that restoring [Ca2+]m homeostasis might prevent or even reverse the effects of HCV.
ER stress
Effects of ER stress in the liver are important for the pathogenesis of a number of metabolic and inflammatory conditions. The ER represents a central regulator of protein folding, quality control, trafficking, and targeting. Therefore, the ability to adapt its capacity to manage synthetic, metabolic, and other unfavorable conditions is extremely important to maintain homeostasis in the cell. Disequilibrium between ER load and folding capacity establishes ER stress, and the ability to adapt to physiological levels of ER stress is important to all cells, but especially to professional secretory cells (156), like hepatocytes (Fig. 2). This adaptation is achieved by the activation of the unfolded protein response (UPR) which consists of the complex coordinated action of three distinct signal transduction pathways mediated by inositol requiring enzyme (IRE) 1α, protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6α (ATF6α) (168, 236). Following activation, UPR signaling pathways act synergistically to encode functions that ameliorate the stressed state of the ER. If ER stress persists, the cell is functionally compromised and signal alarm pathways are activated to lead to apoptosis (168, 236, 335).
UPR activation has been reported in several physiological responses, including glucose and lipid metabolism, inflammation, autophagy, and apoptosis (168). The role of Ca2+ has been particularly noted in the process of ER stress-induced apoptosis. Disequilibrium in ER Ca2+ levels can activate the UPR, and constant or pronounced ER stress leads to apoptosis (168). ER stress-associated cell death results from the action of several apoptosis mediators. Some of these are activated by the UPR sensors, whereas others are related to Ca2+ and redox homeostasis (236). The proapoptotic proteins Bax and Bak as well as the antiapoptotic Bcl-2 that reside in the ER membrane and are known to regulate Ca2+ homeostasis, interact with members of the UPR cascades like IRE1α and c-jun-N-terminal kinase to mediate ER stress-induced apoptosis (162, 210, 401, 416). Furthermore, ER stress-inducing agents lead to sustained Ca2+ release from the ER, accumulation of Ca2+ in the mitochondria and subsequent mitochondrial permeabilization, resulting in release of apoptotic effectors from mitochondria into the cytosol (93). When the antiapoptotic protein Bcl-2 is over expressed, resting free ER Ca2+ and cell death are decreased (119). Moreover, deficiency of the proapoptotic proteins Bak and Bax results in lower free ER Ca2+ content, and reduced sensitivity to apoptotic agents (286). Furthermore, Bax Inhibitor-1 induces Ca2+ release under acidic conditions leading to increased cell death (193). In some cases during UPR activation, translation is resumed prematurely before ER stress is completely resolved. Consequently, newly synthesized proteins can undergo oxidative protein folding in the ER generating ROS (242). Additionally, protein folding in the ER can lead to activation of InsP3Rs and release of [Ca2+]i from the ER, which in turn is transmitted to the mitochondria to produce ROS (298). In both cases, the generation of ROS results in apoptosis. Thus, current evidence suggests that Ca2+ and Ca2+-sensitive proteins play a key role in the development of ER stress-induced apoptosis. However, the exact signaling pathways that mediate this process in hepatocytes and whether ER stress affects the Ca2+ signaling machinery and/or downstream functions of Ca2+ are subjects that need further investigation.
Sustained ER stress and activation of the UPR have also been observed in several liver diseases, and Ca2+ signaling-associated defects have been implicated, in particular, in the progression of obesity and the obesity-related disorder Nonalcoholic fatty liver disease (NAFLD). Indeed, incorporation of saturated fatty acids into the ER disrupt its structure and function (42, 226), affecting the folding capacity and chaperone function by altering ER Ca2+ homeostasis (54, 140, 149, 402). Several lines of evidence suggest that members of the Bcl-2 family of proteins, which directly alter ER Ca2+, may play a direct role in saturated fatty acid-induced hepatocyte cell death and the progression of NAFLD (2, 13, 65, 235, 291). Moreover, palmitic acid requires the flux of Ca2+ and cytochrome C release to mediate ER stress-induced apoptosis (423). Furthermore, a new link has been established between ER stress and obesity, in which protein and mRNA levels of SERCA2b are markedly reduced in the livers of obese mice and recovery of SERCA2b significantly ameliorates ER stress, increases glucose tolerance and achieves euglycemia in severely obese and diabetic mice (293) (Fig. 2). Subsequent studies demonstrated that alterations in the ER fatty acid and lipid composition result in the inhibition of SERCA activity and ER stress. Hence, abnormal lipid and Ca2+ metabolism are important contributors to hepatic ER stress in obesity (123).
Therapeutic agents that affect Ca2+ signaling are currently being used for conditions like spasticity, and other compounds have been tested for specific UPR proteins in the liver (199). However, further investigation is needed to better understand the molecular basis of ER stress-induced liver damage and the precise involvement of Ca2+ signaling in this process to develop targeted therapies for these conditions.
Conclusion
Ca2+ signaling in the liver results from the orchestrated action of multiple members of the Ca2+ signaling machinery in the different cells that form this highly specialized organ. Spatial and temporal Ca2+ signaling patterns should be considered not only at the subcellular and cellular level, but also in the context of the whole organ, in which cells maintain constant communication to achieve the proper performance of liver function. We furthermore are now beginning to understand how defects in Ca2+ signaling at these various levels play a role in the pathogenesis of a wide range of liver diseases. The understanding of how defects in the Ca2+ signaling machinery and in Ca2+ signaling patterns may lead to pathological conditions, may in turn result in the rational design of novel, more specific, and more effective therapeutic approaches.
Acknowledgments
This study was supported by the following NIH Grants: DK57751, DK34989, DK 45710, and DK61747.
References
- 1.Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso MT, Garcia-Sancho J. Nuclear Ca(2+) signalling. Cell Calcium. 2011;49:280–289. doi: 10.1016/j.ceca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, Lesage G, Larusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 5.Alpini G, Lenzi R, Zhai WR, Slott PA, Liu MH, Sarkozi L, Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989;257:G124–G133. doi: 10.1152/ajpgi.1989.257.1.G124. [DOI] [PubMed] [Google Scholar]
- 6.Alvaro D, Alpini G, Jezequel AM, Bassotti C, Francia C, Fraioli F, Romeo R, Marucci L, Le Sage G, Glaser SS, Benedetti A. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest. 1997;100:1349–1362. doi: 10.1172/JCI119655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson PA, Greenberg RM. Phylogeny of ion channels: Clues to structure and function. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:17–28. doi: 10.1016/s1096-4959(01)00376-1. [DOI] [PubMed] [Google Scholar]
- 8.Andrade V, Guerra M, Jardim C, Melo F, Silva W, Ortega JM, Robert M, Nathanson MH, Leite F. Nucleoplasmic calcium regulates cell proliferation through legumain. J Hepatol. 2011;55:626–635. doi: 10.1016/j.jhep.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelis Campos AC, Rodrigues MA, de Andrade C, de Goes AM, Nathanson MH, Gomes DA. Epidermal growth factor receptors destined for the nucleus are internalized via a clathrin-dependent pathway. Biochem Biophys Res Commun. 2011;412:341–346. doi: 10.1016/j.bbrc.2011.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 11.Badminton MN, Campbell AK, Rembold CM. Differential regulation of nuclear and cytosolic Ca2+ in HeLa cells. J Biol Chem. 1996;271:31210–31214. doi: 10.1074/jbc.271.49.31210. [DOI] [PubMed] [Google Scholar]
- 12.Baffy G, Yang LJ, Michalopoulos GK, Williamson JR. Hepatocyte growth-factor induces calcium mobilization and inositol phosphate production in rat hepatocytes. J Cell Physiol. 1992;153:332–339. doi: 10.1002/jcp.1041530213. [DOI] [PubMed] [Google Scholar]
- 13.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 14.Barritt GJ, Chen J, Rychkov GY. Ca(2+)-permeable channels in the hepatocyte plasma membrane and their roles in hepatocyte physiology. Biochim Biophys Acta. 2008;1783:651–672. doi: 10.1016/j.bbamcr.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Barritt GJ, Litjens TL, Castro J, Aromataris E, Rychkov GY. Store-operated Ca2+ channels and microdomains of Ca2+ in liver cells. Clin Exp Pharmacol Physiol. 2009;36:77–83. doi: 10.1111/j.1440-1681.2008.05095.x. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett PJ, Young KW, Nahorski SR, Challiss RA. Single cell analysis and temporal profiling of agonist-mediated inositol 1,4,5-trisphosphate, Ca2+, diacylglycerol, and protein kinase C signaling using fluorescent biosensors. J Biol Chem. 2005;280:21837–21846. doi: 10.1074/jbc.M411843200. [DOI] [PubMed] [Google Scholar]
- 17.Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 2004;23:1207–1216. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, Buscail L, Bartenschlager R, Ichas F, Rizzuto R, Paterlini-Brechot P. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24:4921–4933. doi: 10.1038/sj.onc.1208673. [DOI] [PubMed] [Google Scholar]
- 20.Bernardi P. Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 21.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernardi P, Rasola A. Calcium and cell death: The mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 23.Berridge MJ. Inositol trisphosphate and calcium signaling. Ann N Y Acad Sci. 1995;766:31–43. doi: 10.1111/j.1749-6632.1995.tb26646.x. [DOI] [PubMed] [Google Scholar]
- 24.Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499 (Pt 2):291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 26.Berridge MJ, Galione A. Cytosolic calcium oscillators. FASEB J. 1988;2:3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- 27.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 28.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 29.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206–1216. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- 30.Beuers U, Nathanson MH, Boyer JL. Effects of tauroursodeoxycholic acid on cytosolic Ca2+ signals in isolated rat hepatocytes. Gastroenterology. 1993;104:604–612. doi: 10.1016/0016-5085(93)90433-d. [DOI] [PubMed] [Google Scholar]
- 31.Beuers U, Nathanson MH, Isales CM, Boyer JL. Tauroursodeoxycholic acid stimulates hepatocellular exocytosis and mobilizes extracellular Ca++ mechanisms defective in cholestasis. J Clin Invest. 1993;92:2984–2993. doi: 10.1172/JCI116921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 33.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 34.Blackmore PF, Assimacopoulos-Jeannet F, Chan TM, Exton JH. Studies on alpha-adrenergic activation of hepatic glucose output. Insulin inhibition of alpha-adrenergic and glucagon actions in normal and calcium-depleted hepatocytes. J Biol Chem. 1979;254:2828–2834. [PubMed] [Google Scholar]
- 35.Blackmore PF, Strickland WG, Bocckino SB, Exton JH. Mechanism of hepatic glycogen synthase inactivation induced by Ca2+-mobilizing hormones. Studies using phospholipase C and phorbol myristate acetate. Biochem J. 1986;237:235–242. doi: 10.1042/bj2370235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 37.Boehning D, van Rossum DB, Patterson RL, Snyder SH. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc Natl Acad Sci U S A. 2005;102:1466–1471. doi: 10.1073/pnas.0409650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boitano S, Dirksen ER, Evans WH. Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium. 1998;23:1–9. doi: 10.1016/s0143-4160(98)90069-0. [DOI] [PubMed] [Google Scholar]
- 39.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 40.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Bootman MD, Berridge MJ, Roderick HL. Calcium signalling: More messengers, more channels, more complexity. Curr Biol. 2002;12:R563–R565. doi: 10.1016/s0960-9822(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 42.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Bouchard MJ, Puro RJ, Wang L, Schneider RJ. Activation and inhibition of cellular calcium and tyrosine kinase signaling pathways identify targets of the HBx protein involved in hepatitis B virus replication. J Virol. 2003;77:7713–7719. doi: 10.1128/JVI.77.14.7713-7719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725–12734. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouchard MJ, Wang L, Schneider RJ. Activation of focal adhesion kinase by hepatitis B virus HBx protein: Multiple functions in viral replication. J Virol. 2006;80:4406–4414. doi: 10.1128/JVI.80.9.4406-4414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001;294:2376–2378. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- 47.Boucherie S, Koukoui O, Nicolas V, Combettes L. Cholestatic bile acids inhibit gap junction permeability in rat hepatocyte couplets and normal rat cholangiocytes. J Hepatol. 2005;42:244–251. doi: 10.1016/j.jhep.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Bouscarel B, Fromm H, Nussbaum R. Ursodeoxycholate mobilizes intracellular Ca2+ and activates phosphorylase a in isolated hepatocytes. Am J Physiol. 1993;264:G243–G251. doi: 10.1152/ajpgi.1993.264.2.G243. [DOI] [PubMed] [Google Scholar]
- 49.Bradford PG, Maglich JM, Kirkwood KL. IL-1 beta increases type 1 inositol trisphosphate receptor expression and IL-6 secretory capacity in osteoblastic cell cultures. Mol Cell Biol Res Commun. 2000;3:73–75. doi: 10.1006/mcbr.2000.0194. [DOI] [PubMed] [Google Scholar]
- 50.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brini M, Murgia M, Pasti L, Picard D, Pozzan T, Rizzuto R. Nuclear Ca2+ concentration measured with specifically targeted recombinant aequorin. EMBO J. 1993;12:4813–4819. doi: 10.1002/j.1460-2075.1993.tb06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruce JI, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 53.Bruck R, Nathanson MH, Roelofsen H, Boyer JL. Effects of protein kinase C and cytosolic Ca2+ on exocytosis in the isolated perfused rat liver. Hepatology. 1994;20:1032–1040. doi: 10.1002/hep.1840200436. [DOI] [PubMed] [Google Scholar]
- 54.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Burgess GM, Bird GS, Obie JF, Putney JW., Jr The mechanism for synergism between phospholipase C- and adenylylcyclase-linked hormones in liver. Cyclic AMP-dependent kinase augments inositol trisphosphate-mediated Ca2+ mobilization without increasing the cellular levels of inositol polyphosphates. J Biol Chem. 1991;266:4772–4781. [PubMed] [Google Scholar]
- 56.Burgstahler AD, Nathanson MH. Coordination of calcium waves among hepatocytes: Teamwork gets the job done. Hepatology. 1998;27:634–635. doi: 10.1002/hep.510270244. [DOI] [PubMed] [Google Scholar]
- 57.Bustamante JO, Michelette ER, Geibel JP, Dean DA, Hanover JA, McDonnell TJ. Calcium, ATP and nuclear pore channel gating. Pflugers Arch. 2000;439:433–444. doi: 10.1007/s004249900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 60.Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 61.Carafoli E. The fateful encounter of mitochondria with calcium: How did it happen? Biochim Biophys Acta. 2010;1797:595–606. doi: 10.1016/j.bbabio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 62.Cardenas C, Escobar M, Garcia A, Osorio-Reich M, Hartel S, Foskett JK, Franzini-Armstrong C. Visualization of inositol 1,4,5-trisphosphate receptors on the nuclear envelope outer membrane by freeze-drying and rotary shadowing for electron microscopy. J Struct Biol. 2010;171:372–381. doi: 10.1016/j.jsb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 64.Carver RS, Stevenson MC, Scheving LA, Russell WE. Diverse expression of ErbB receptor proteins during rat liver development and regeneration. Gastroenterology. 2002;123:2017–2027. doi: 10.1053/gast.2002.37060. [DOI] [PubMed] [Google Scholar]
- 65.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, Gores GJ. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chami M, Oules B, Paterlini-Brechot P. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim Biophys Acta. 2006;1763:1344–1362. doi: 10.1016/j.bbamcr.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 67.Charest R, Blackmore PF, Berthon B, Exton JH. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983;258:8769–8773. [PubMed] [Google Scholar]
- 68.Chen HS, Kaneko S, Girones R, Anderson RW, Hornbuckle WE, Tennant BC, Cote PJ, Gerin JL, Purcell RH, Miller RH. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J Virol. 1993;67:1218–1226. doi: 10.1128/jvi.67.3.1218-1226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: Sometimes good and sometimes bad teamwork. Sci STKE. 2006;(363):re15. doi: 10.1126/stke.3632006re15. [DOI] [PubMed] [Google Scholar]
- 70.Choi Y, Gyoo PS, Yoo JH, Jung G. Calcium ions affect the hepatitis B virus core assembly. Virology. 2005;332:454–463. doi: 10.1016/j.virol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 71.Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 72.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 73.Clark EA, Brugge JS. Integrins and signal transduction pathways: The road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 74.Cocco L, Faenza I, Fiume R, Maria BA, Gilmour RS, Manzoli FA. Phosphoinositide-specific phospholipase C (PI-PLC) beta1 and nuclear lipid-dependent signaling. Biochim Biophys Acta. 2006;1761:509–521. doi: 10.1016/j.bbalip.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Combettes L, Berthon B, Doucet E, Erlinger S, Claret M. Bile acids mobilise internal Ca2 +independently of external Ca2+ in rat hepatocytes. Eur J Biochem. 1990;190:619–623. doi: 10.1111/j.1432-1033.1990.tb15617.x. [DOI] [PubMed] [Google Scholar]
- 76.Combettes L, Dumont M, Berthon B, Erlinger S, Claret M. Release of calcium from the endoplasmic reticulum by bile acids in rat liver cells. J Biol Chem. 1988;263:2299–2303. [PubMed] [Google Scholar]
- 77.Combettes L, Tran D, Tordjmann T, Laurent M, Berthon B, Claret M. Ca(2+)-mobilizing hormones induce sequentially ordered Ca2+ signals in multicellular systems of rat hepatocytes. Biochem J. 1994;304 (Pt 2):585–594. doi: 10.1042/bj3040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Correa PR, Guerra MT, Leite MF, Spray DC, Nathanson MH. Endotoxin unmasks the role of gap junctions in the liver. Biochem Biophys Res Commun. 2004;322:718–726. doi: 10.1016/j.bbrc.2004.07.192. [DOI] [PubMed] [Google Scholar]
- 79.Craske M, Takeo T, Gerasimenko O, Vaillant C, Torok K, Petersen OH, Tepikin AV. Hormone-induced secretory and nuclear translocation of calmodulin: Oscillations of calmodulin concentration with the nucleus as an integrator. Proc Natl Acad Sci U S A. 1999;96:4426–4431. doi: 10.1073/pnas.96.8.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341 (Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 81.Crompton M, Kunzi M, Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur J Biochem. 1977;79:549–558. doi: 10.1111/j.1432-1033.1977.tb11839.x. [DOI] [PubMed] [Google Scholar]
- 82.Cruise JL, Muga SJ, Lee YS, Michalopoulos GK. Regulation of hepatocyte growth - alpha-1 adrenergic-receptor and Ras P21 changes in liver-regeneration. J Cell Physiol. 1989;140:195–201. doi: 10.1002/jcp.1041400202. [DOI] [PubMed] [Google Scholar]
- 83.Cruz LN, Guerra MT, Kruglov E, Mennone A, Garcia CR, Chen J, Nathanson MH. Regulation of multidrug resistance-associated protein 2 by calcium signaling in mouse liver. Hepatology. 2010;52:327–337. doi: 10.1002/hep.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, Schirmacher P, Biesterfeld S, Galle PR, Lohse AW, Kanzler S. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419–428. doi: 10.1016/j.jhep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 87.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 88.De Young GW, Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992;89:9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dehaye JP, Hughes BP, Blackmore PF, Exton JH. Insulin inhibition of alpha-adrenergic actions in liver. Biochem J. 1981;194:949–956. doi: 10.1042/bj1940949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 91.Deisseroth K, Tsien RW. Dynamic multiphosphorylation passwords for activity-dependent gene expression. Neuron. 2002;34:179–182. doi: 10.1016/s0896-6273(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 92.Dellis O, Dedos SG, Tovey SC, Taufiq UR, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- 93.Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 94.Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 95.Diaz-Munoz M, Canedo-Merino R, Gutierrez-Salinas J, Hernandez-Munoz R. Modifications of intracellular calcium release channels and calcium mobilization following 70% hepatectomy. Arch Biochem Biophys. 1998;349:105–112. doi: 10.1006/abbi.1997.0396. [DOI] [PubMed] [Google Scholar]
- 96.Dixon CJ, Hall JF, Webb TE, Boarder MR. Regulation of rat hepatocyte function by P2Y receptors: Focus on control of glycogen phosphorylase and cyclic AMP by 2-methylthioadenosine 5′-diphosphate. J Pharmacol Exp Ther. 2004;311:334–341. doi: 10.1124/jpet.104.067744. [DOI] [PubMed] [Google Scholar]
- 97.Dixon CJ, White PJ, Hall JF, Kingston S, Boarder MR. Regulation of human hepatocytes by P2Y receptors: Control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J Pharmacol Exp Ther. 2005;313:1305–1313. doi: 10.1124/jpet.104.082743. [DOI] [PubMed] [Google Scholar]
- 98.Dixon CJ, Woods NM, Webb TE, Green AK. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br J Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 100.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 101.Dranoff JA, Masyuk AI, Kruglov EA, Larusso NF, Nathanson MH. Polarized expression and function of P2Y ATP receptors in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1059–G1067. doi: 10.1152/ajpgi.2001.281.4.G1059. [DOI] [PubMed] [Google Scholar]
- 102.Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–G424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dufour JF, Luthi M, Forestier M, Magnino F. Expression of inositol 1,4,5-trisphosphate receptor isoforms in rat cirrhosis. Hepatology. 1999;30:1018–1026. doi: 10.1002/hep.510300421. [DOI] [PubMed] [Google Scholar]
- 104.DuPont G, Combettes L, Leybaert L. Calcium dynamics: Spatio-temporal organization from the subcellular to the organ level. Int Rev Cytol. 2007;261:193–245. doi: 10.1016/S0074-7696(07)61005-5. [DOI] [PubMed] [Google Scholar]
- 105.DuPont G, Swillens S, Clair C, Tordjmann T, Combettes L. Hierarchical organization of calcium signals in hepatocytes: From experiments to models. Biochim Biophys Acta. 2000;1498:134–152. doi: 10.1016/s0167-4889(00)00090-2. [DOI] [PubMed] [Google Scholar]
- 106.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eugenin EA, Gonzalez H, Saez CG, Saez JC. Gap junctional communication coordinates vasopressin-induced glycogenolysis in rat hepatocytes. Am J Physiol. 1998;274:G1109–G1116. doi: 10.1152/ajpgi.1998.274.6.G1109. [DOI] [PubMed] [Google Scholar]
- 108.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 109.Exton JH. Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab Rev. 1987;3:163–183. doi: 10.1002/dmr.5610030108. [DOI] [PubMed] [Google Scholar]
- 110.Failli P, Ruocco C, De Franco R, Caligiuri A, Gentilini A, Giotti A, Gentilini P, Pinzani M. The mitogenic effect of platelet-derived growth factor in human hepatic stellate cells requires calcium influx. Am J Physiol. 1995;269:C1133–C1139. doi: 10.1152/ajpcell.1995.269.5.C1133. [DOI] [PubMed] [Google Scholar]
- 111.Fallon MB, Nathanson MH, Mennone A, Saez JC, Burgstahler AD, Anderson JM. Altered expression and function of hepatocyte gap junctions after common bile duct ligation in the rat. Am J Physiol. 1995;268:C1186–C1194. doi: 10.1152/ajpcell.1995.268.5.C1186. [DOI] [PubMed] [Google Scholar]
- 112.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 113.Feng L, Krausfriedmann N. Changes in 1,4,5-inositol trisphosphate binding following partial-hepatectomy. Biochem Biophys Res Commun. 1994;205:291–297. doi: 10.1006/bbrc.1994.2663. [DOI] [PubMed] [Google Scholar]
- 114.Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5–trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 115.Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M. Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology. 2007;133:1603–1613. doi: 10.1053/j.gastro.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 116.Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993;91:319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Foskett JK, Roifman CM, Wong D. Activation of calcium oscillations by thapsigargin in parotid acinar cells. J Biol Chem. 1991;266:2778–2782. [PubMed] [Google Scholar]
- 118.Fox JL, Burgstahler AD, Nathanson MH. Mechanism of long-range Ca2+ signalling in the nucleus of isolated rat hepatocytes. Biochem J. 1997;326 (Pt 2):491–495. doi: 10.1042/bj3260491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Francis H, Glaser S, DeMorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 122.Francis HL, DeMorrow S, Franchitto A, Venter JK, Mancinelli RA, White MA, Meng F, Ueno Y, Carpino G, Renzi A, Baker KK, Shine HE, Francis TC, Gaudio E, Alpini GD, Onori P. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP(3)/Ca(2+) and cAMP-dependent signaling mechanisms. Lab Invest. 2012;92:282–294. doi: 10.1038/labinvest.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fukuda K, Yamamoto M. Acquisition of resistance to apoptosis and necrosis by Bcl-xL over-expression in rat hepatoma McA-RH8994 cells. J Gastroenterol Hepatol. 1999;14:682–690. doi: 10.1046/j.1440-1746.1999.01935.x. [DOI] [PubMed] [Google Scholar]
- 125.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 126.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 127.Galione A, Churchill GC. Interactions between calcium release pathways: Multiple messengers and multiple stores. Cell Calcium. 2002;32:343–354. doi: 10.1016/s0143416002001902. [DOI] [PubMed] [Google Scholar]
- 128.Galione A, Petersen OH. The NAADP receptor: New receptors or new regulation? Mol Interv. 2005;5:73–79. doi: 10.1124/mi.5.2.4. [DOI] [PubMed] [Google Scholar]
- 129.Gaspers LD, Thomas AP. Calcium signaling in liver. Cell Calcium. 2005;38:329–342. doi: 10.1016/j.ceca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 130.Gearhart TL, Bouchard MJ. Replication of the hepatitis B virus requires a calcium-dependent HBx-induced G1 phase arrest of hepatocytes. Virology. 2010a;407:14–25. doi: 10.1016/j.virol.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gearhart TL, Bouchard MJ. The hepatitis B virus X protein modulates hepatocyte proliferation pathways to stimulate viral replication. J Virol. 2010b;84:2675–2686. doi: 10.1128/JVI.02196-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gerasimenko O, Gerasimenko J. New aspects of nuclear calcium signalling. J Cell Sci. 2004;117:3087–3094. doi: 10.1242/jcs.01295. [DOI] [PubMed] [Google Scholar]
- 133.Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- 134.Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. Calcium transport pathways in the nucleus. Pflugers Arch. 1996;432:1–6. doi: 10.1007/s004240050098. [DOI] [PubMed] [Google Scholar]
- 135.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: A possible regulatory mechanism in mitochondrial function. Biochem J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem. 2008;283:4344–4351. doi: 10.1074/jbc.M706550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gonzales E, Julien B, Serriere-Lanneau V, Nicou A, Doignon I, Lagoudakis L, Garcin I, Azoulay D, Duclos-Vallee JC, Castaing D, Samuel D, Hernandez-Garcia A, Awad SS, Combettes L, Thevananther S, Tordjmann T. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol. 2010;52:54–62. doi: 10.1016/j.jhep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gonzalez HE, Eugenin EA, Garces G, Solis N, Pizarro M, Accatino L, Saez JC. Regulation of hepatic connexins in cholestasis: Possible involvement of Kupffer cells and inflammatory mediators. Am J Physiol Gastrointest Liver Physiol. 2002;282:G991–G1001. doi: 10.1152/ajpgi.00298.2001. [DOI] [PubMed] [Google Scholar]
- 140.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: Folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 141.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci U S A. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grimm S, Brdiczka D. The permeability transition pore in cell death. Apoptosis. 2007;12:841–855. doi: 10.1007/s10495-007-0747-3. [DOI] [PubMed] [Google Scholar]
- 143.Groigno L, Whitaker M. An anaphase calcium signal controls chromosome disjunction in early sea urchin embryos. Cell. 1998;92:193–204. doi: 10.1016/s0092-8674(00)80914-9. [DOI] [PubMed] [Google Scholar]
- 144.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 145.Guerra MT, Fonseca EA, Melo FM, Andrade VA, Aguiar CJ, Andrade LM, Pinheiro ACN, Casteluber MCF, Resende RR, Pinto MCX, Fernandes SOA, Cardoso VN, Souza-Fagundes EM, Menezes GB, de Paula AM, Nathanson MH, Leite MD. Mitochondrial calcium regulates rat liver regeneration through the modulation of apoptosis. Hepatology. 2011;54:296–306. doi: 10.1002/hep.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: Physiological and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 147.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 148.Guo YS, Tang J, Chen B, Huang W, Li Y, Cui HY, Zhang X, Wang SJ, Chen ZN, Jiang JL. ssig-h3 regulates store-operated Ca(2+) entry and promotes the invasion of human hepatocellular carcinoma cells. Cell Biol Int. 2011;35:811–817. doi: 10.1042/CBI20100916. [DOI] [PubMed] [Google Scholar]
- 149.Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–E701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 150.Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hagar RE, Ehrlich BE. Regulation of the type III InsP(3) receptor by InsP(3) and ATP. Biophys J. 2000;79:271–278. doi: 10.1016/S0006-3495(00)76289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hajnoczky G, Csordas G. Calcium signalling: Fishing out molecules of mitochondrial calcium transport. Curr Biol. 2010;20:R888–R891. doi: 10.1016/j.cub.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 154.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 155.Hansen CA, Yang LJ, Williamson JR. Mechanisms of receptor-mediated Ca2+ signaling in rat hepatocytes. J Biol Chem. 1991;266:18573–18579. [PubMed] [Google Scholar]
- 156.Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: A review. Diabetes. 2002;51 (Suppl 3):S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- 157.Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 158.Hayashi PH, Di Bisceglie AM. The progression of hepatitis B- and C-infections to chronic liver disease and hepatocellular carcinoma: Epidemiology and pathogenesis. Med Clin North Am. 2005;89:371–389. doi: 10.1016/j.mcna.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 159.Hems DA, Whitton PD. Control of hepatic glycogenolysis. Physiol Rev. 1980;60:1–50. doi: 10.1152/physrev.1980.60.1.1. [DOI] [PubMed] [Google Scholar]
- 160.Hennager DJ, Welsh MJ, DeLisle S. Changes in either cytosolic or nucleoplasmic inositol 1,4,5-trisphosphate levels can control nuclear Ca2+ concentration. J Biol Chem. 1995;270:4959–4962. doi: 10.1074/jbc.270.10.4959. [DOI] [PubMed] [Google Scholar]
- 161.Hernandez E, Leite MF, Guerra MT, Kruglov EA, Bruna-Romero O, Rodrigues MA, Gomes DA, Giordano FJ, Dranoff JA, Nathanson MH. The spatial distribution of inositol 1,4,5-trisphosphate receptor isoforms shapes Ca2+ waves. J Biol Chem. 2007;282:10057–10067. doi: 10.1074/jbc.M700746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 163.Hirata K, Dufour JF, Shibao K, Knickelbein R, O’Neill AF, Bode HP, Cassio D, St Pierre MV, Larusso NF, Leite MF, Nathanson MH. Regulation of Ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002;36:284–296. doi: 10.1053/jhep.2002.34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hirata K, Nathanson MH. Bile duct epithelia regulate biliary bicarbonate excretion in normal rat liver. Gastroenterology. 2001;121:396–406. doi: 10.1053/gast.2001.26280. [DOI] [PubMed] [Google Scholar]
- 165.Hirata K, Pusl T, O’Neill AF, Dranoff JA, Nathanson MH. The type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088–1100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 166.Holzinger F, Schteingart CD, Ton-Nu HT, Eming SA, Monte MJ, Hagey LR, Hofmann AF. Fluorescent bile acid derivatives: Relationship between chemical structure and hepatic and intestinal transport in the rat. Hepatology. 1997;26:1263–1271. doi: 10.1002/hep.510260526. [DOI] [PubMed] [Google Scholar]
- 167.Hoppe UC. Mitochondrial calcium channels. FEBS Lett. 2010;584:1975–1981. doi: 10.1016/j.febslet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 168.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huertabahena J, Villalobosmolina R, Corvera S, Garciasainz JA. Sensitivity of liver-cells formed after partial-hepatectomy to glucagon, vasopressin and angiotensin-II. Biochim Biophys Acta. 1983;763:120–124. doi: 10.1016/0167-4889(83)90034-4. [DOI] [PubMed] [Google Scholar]
- 170.Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Humbert JP, Matter N, Artault JC, Koppler P, Malviya AN. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J Biol Chem. 1996;271:478–485. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- 172.Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci U S A. 2005;102:14386–14391. doi: 10.1073/pnas.0503215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 174.Ichas F, Jouaville LS, Sidash SS, Mazat JP, Holmuhamedov EL. Mitochondrial calcium spiking: A transduction mechanism based on calcium-induced permeability transition involved in cell calcium signalling. FEBS Lett. 1994;348:211–215. doi: 10.1016/0014-5793(94)00615-6. [DOI] [PubMed] [Google Scholar]
- 175.Iizuka M, Murata T, Hori M, Ozaki H. Increased contractility of hepatic stellate cells in cirrhosis is mediated by enhanced Ca2+-dependent and Ca2+-sensitization pathways. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1010–G1021. doi: 10.1152/ajpgi.00350.2010. [DOI] [PubMed] [Google Scholar]
- 176.Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol. 2003;4:349–360. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 177.Irvine RF. Nuclear inositide signalling – expansion, structures and clarification. Biochim Biophys Acta. 2006;1761:505–508. doi: 10.1016/j.bbalip.2006.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem Biophys Res Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- 179.Jafri MS, Keizer J. Diffusion of inositol 1,4,5-trisphosphate but not Ca2+ is necessary for a class of inositol 1,4,5-trisphosphate-induced Ca2+ waves. Proc Natl Acad Sci U S A. 1994;91:9485–9489. doi: 10.1073/pnas.91.20.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jiang QX, Thrower EC, Chester DW, Ehrlich BE, Sigworth FJ. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 A resolution. EMBO J. 2002;21:3575–3581. doi: 10.1093/emboj/cdf380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Joffre C, Barrow R, Menard L, Calleja V, Hart IR, Kermorgant S. A direct role for Met endocytosis in tumorigenesis. Nat Cell Biol. 2011;13:827–837. doi: 10.1038/ncb2257. [DOI] [PubMed] [Google Scholar]
- 182.Jones BF, Boyles RR, Hwang SY, Bird GS, Putney JW. Calcium influx mechanisms underlying calcium oscillations in rat hepatocytes. Hepatology. 2008;48:1273–1281. doi: 10.1002/hep.22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 184.Jungermann K, Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69:708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- 185.Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev. 2003;24:719–736. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- 186.Kanno N, Lesage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–G625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 187.Kasai H, Augustine GJ. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990;348:735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- 188.Katz A, Wu D, Simon MI. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992;360:686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- 189.Kawanishi T, Blank LM, Harootunian AT, Smith MT, Tsien RY. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989;264:12859–12866. [PubMed] [Google Scholar]
- 190.Keppens S, De Wulf H. Characterization of the liver P2-purinoceptor involved in the activation of glycogen phosphorylase. Biochem J. 1986;240:367–371. doi: 10.1042/bj2400367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khan MT, Wagner L, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2006;281:3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- 192.Khoo KM, Han MK, Park JB, Chae SW, Kim UH, Lee HC, Bay BH, Chang CF. Localization of the cyclic ADP-ribose-dependent calcium signaling pathway in hepatocyte nucleus. J Biol Chem. 2000;275:24807–24817. doi: 10.1074/jbc.M908231199. [DOI] [PubMed] [Google Scholar]
- 193.Kim HR, Lee GH, Ha KC, Ahn T, Moon JY, Lee BJ, Cho SG, Kim S, Seo YR, Shin YJ, Chae SW, Reed JC, Chae HJ. Bax Inhibitor-1 Is a pH-dependent regulator of Ca2+ channel activity in the endoplasmic reticulum. J Biol Chem. 2008;283:15946–15955. doi: 10.1074/jbc.M800075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim SY, Cho BH, Kim UH. CD38-mediated Ca2+ signaling contributes to angiotensin II-induced activation of hepatic stellate cells: Attenuation of hepatic fibrosis by CD38 ablation. J Biol Chem. 2010;285:576–582. doi: 10.1074/jbc.M109.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 2011;1813:616–622. doi: 10.1016/j.bbamcr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kitamura T, Brauneis U, Gatmaitan Z, Arias IM. Extracellular ATP, intracellular calcium and canalicular contraction in rat hepatocyte doublets. Hepatology. 1991;14:640–647. doi: 10.1016/0270-9139(91)90051-v. [DOI] [PubMed] [Google Scholar]
- 197.Kitamura T, Watanabe S, Ikejima KI, Hirose M, Miyazaki A, Yumoto A, Suzuki S, Yamada T, Kitami N, Sato N. Different features of Ca2+ oscillations in differentiated and undifferentiated hepatocyte doublets. Hepatology. 1995;21:1395–1404. [PubMed] [Google Scholar]
- 198.Kojima N, Hori M, Murata T, Morizane Y, Ozaki H. Different profiles of Ca2+ responses to endothelin-1 and PDGF in liver myofibroblasts during the process of cell differentiation. Br J Pharmacol. 2007;151:816–827. doi: 10.1038/sj.bjp.0707269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kraskiewicz H, FitzGerald U. InterfERing with endoplasmic reticulum stress. Trends Pharmacol Sci. 2012;33:53–63. doi: 10.1016/j.tips.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 200.Krebs EG. The Albert Lasker Medical Awards. Role of the cyclic AMP-dependent protein kinase in signal transduction. JAMA. 1989;262:1815–1818. doi: 10.1001/jama.262.13.1815. [DOI] [PubMed] [Google Scholar]
- 201.Kruglov EA, Correa PR, Arora G, Yu J, Nathanson MH, Dranoff JA. Molecular basis for calcium signaling in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G975–G982. doi: 10.1152/ajpgi.00401.2006. [DOI] [PubMed] [Google Scholar]
- 202.Kruglov EA, Gautam S, Guerra MT, Nathanson MH. Type 2 inositol 1,4,5-trisphosphate receptor modulates bile salt export pump activity in rat hepatocytes. Hepatology. 2011;54:1790–1799. doi: 10.1002/hep.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kullak-Ublick GA, Meier PJ. Mechanisms of cholestasis. Clin Liver Dis. 2000;4:357–385. doi: 10.1016/s1089-3261(05)70114-8. [DOI] [PubMed] [Google Scholar]
- 204.Kumar NM, Gilula NB. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986;103:767–776. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kupzig S, Deaconescu D, Bouyoucef D, Walker SA, Liu Q, Polte CL, Daumke O, Ishizaki T, Lockyer PJ, Wittinghofer A, Cullen PJ. GAP1 family members constitute bifunctional Ras and Rap GTPase-activating proteins. J Biol Chem. 2006;281:9891–9900. doi: 10.1074/jbc.M512802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lagoudakis L, Garcin I, Julien B, Nahum K, Gomes DA, Combettes L, Nathanson MH, Tordjmann T. Cytosolic calcium regulates liver regeneration in the rat. Hepatology. 2010;52:602–611. doi: 10.1002/hep.23673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lanini L, Bachs O, Carafoli E. The calcium pump of the liver nuclear membrane is identical to that of endoplasmic reticulum. J Biol Chem. 1992;267:11548–11552. [PubMed] [Google Scholar]
- 208.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: Disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 209.Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- 210.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Leite FM, Guerra MT, Nathanson MH. Ca2+ Signaling in the Liver. In: Arias I, Wolkoff A, Boyer J, Shafritz D, Fausto N, Alter H, Cohen D, editors. The Liver: Biology and Pathobiology. John Wiley & Sons, Ltd; 2009. pp. 485–510. [Google Scholar]
- 212.Leite MF, Burgstahler AD, Nathanson MH. Ca2+ waves require sequential activation of inositol trisphosphate receptors and ryanodine receptors in pancreatic acini. Gastroenterology. 2002;122:415–427. doi: 10.1053/gast.2002.30982. [DOI] [PubMed] [Google Scholar]
- 213.Leite MF, Dranoff JA, Gao L, Nathanson MH. Expression and subcellular localization of the ryanodine receptor in rat pancreatic acinar cells. Biochem J. 1999;337 (Pt 2):305–309. [PMC free article] [PubMed] [Google Scholar]
- 214.Leite MF, Hirata K, Pusl T, Burgstahler AD, Okazaki K, Ortega JM, Goes AM, Prado MA, Spray DC, Nathanson MH. Molecular basis for pacemaker cells in epithelia. J Biol Chem. 2002;277:16313–16323. doi: 10.1074/jbc.M109207200. [DOI] [PubMed] [Google Scholar]
- 215.Leite MF, Thrower EC, Echevarria W, Koulen P, Hirata K, Bennett AM, Ehrlich BE, Nathanson MH. Nuclear and cytosolic calcium are regulated independently. Proc Natl Acad Sci U S A. 2003;100:2975–2980. doi: 10.1073/pnas.0536590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–G190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 218.LeSage GD, Alvaro D, Glaser S, Francis H, Marucci L, Roskams T, Phinizy JL, Marzioni M, Benedetti A, Taffetani S, Barbaro B, Fava G, Ueno Y, Alpini G. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116–1127. doi: 10.1002/hep.20424. [DOI] [PubMed] [Google Scholar]
- 219.LeSage GD, Glaser SS, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, Papa E, Tretjak Z, Jezequel AM, Holcomb LA, Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol. 1999;276:G1289–G1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 220.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 221.Li C, Fox CJ, Master SR, Bindokas VP, Chodosh LA, Thompson CB. Bcl-X(L) affects Ca(2+) homeostasis by altering expression of inositol 1,4,5-trisphosphate receptors. Proc Natl Acad Sci U S A. 2002;99:9830–9835. doi: 10.1073/pnas.152571899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 223.Lin C, Hajnoczky G, Thomas AP. Propagation of cytosolic calcium waves into the nuclei of hepatocytes. Cell Calcium. 1994;16:247–258. doi: 10.1016/0143-4160(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 224.Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lipp P, Thomas D, Berridge MJ, Bootman MD. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J. 1997;16:7166–7173. doi: 10.1093/emboj/16.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 228.Llovet JM, Beaugrand M. Hepatocellular carcinoma: Present status and future prospects. J Hepatol. 2003;38:S136–S149. doi: 10.1016/s0168-8278(02)00432-4. [DOI] [PubMed] [Google Scholar]
- 229.Lowe PJ, Miyai K, Steinbach JH, Hardison WG. Hormonal regulation of hepatocyte tight junctional permeability. Am J Physiol. 1988;255:G454–G461. doi: 10.1152/ajpgi.1988.255.4.G454. [DOI] [PubMed] [Google Scholar]
- 230.Lui PP, Kong SK, Fung KP, Lee CY. The rise of nuclear and cytosolic Ca2+ can be uncoupled in HeLa cells. Pflugers Arch. 1998;436:371–376. doi: 10.1007/s004240050645. [DOI] [PubMed] [Google Scholar]
- 231.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 232.Magnino F, Luthi M, Schmidt K, Dufour JF. The calcium signaling machinery during hepatic regeneration. J Hepatol. 2000;32:78. [Google Scholar]
- 233.Mak DO, Foskett JK. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J Biol Chem. 1994;269:29375–29378. [PubMed] [Google Scholar]
- 234.Malhas A, Goulbourne C, Vaux DJ. The nucleoplasmic reticulum: Form and function. Trends Cell Biol. 2011;21:362–373. doi: 10.1016/j.tcb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 235.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 236.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Malli R, Graier WF. Mitochondrial Ca2+ channels: Great unknowns with important functions. FEBS Lett. 2010;584:1942–1947. doi: 10.1016/j.febslet.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Malviya AN, Klein C. Mechanism regulating nuclear calcium signaling. Can J Physiol Pharmacol. 2006;84:403–422. doi: 10.1139/y05-130. [DOI] [PubMed] [Google Scholar]
- 239.Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, Venter J, DeMorrow S, Carpino G, Kopriva S, White M, Fava G, Alvaro D, Alpini G. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Maranto AR. Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J Biol Chem. 1994;269:1222–1230. [PubMed] [Google Scholar]
- 241.Marchenko SM, Yarotskyy VV, Kovalenko TN, Kostyuk PG, Thomas RC. Spontaneously active and InsP3-activated ion channels in cell nuclei from rat cerebellar Purkinje and granule neurones. J Physiol. 2005;565:897–910. doi: 10.1113/jphysiol.2004.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Marzioni M, Francis H, Benedetti A, Ueno Y, Fava G, Venter J, Reichenbach R, Mancino MG, Summers R, Alpini G, Glaser S. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tau-roursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006;168:398–409. doi: 10.2353/ajpath.2006.050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, Larusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2 +and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Matsu-ura T, Michikawa T, Inoue T, Miyawaki A, Yoshida M, Mikoshiba K. Cytosolic inositol 1,4,5-trisphosphate dynamics during intracellular calcium oscillations in living cells. J Cell Biol. 2006;173:755–765. doi: 10.1083/jcb.200512141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 248.Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, Minowa O, Kuno J, Sakakibara S, Yamada M, Yoneshima H, Miyawaki A, Fukuuchi Y, Furuichi T, Okano H, Mikoshiba K, Noda T. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- 249.Mauger JP. Role of the nuclear envelope in calcium signalling. Biol Cell. 2012;104:70–83. doi: 10.1111/boc.201100103. [DOI] [PubMed] [Google Scholar]
- 250.McGill JM, Basavappa S, Mangel AW, Shimokura GH, Middleton JP, Fitz JG. Adenosine triphosphate activates ion permeabilities in biliary epithelial cells. Gastroenterology. 1994;107:236–243. doi: 10.1016/0016-5085(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 251.McPherson PS, Campbell KP. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993;268:13765–13768. [PubMed] [Google Scholar]
- 252.Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: A view from the lumen. Trends Biochem Sci. 1998;23:10–14. doi: 10.1016/s0968-0004(97)01143-2. [DOI] [PubMed] [Google Scholar]
- 253.Meszaros LG, Bak J, Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature. 1993;364:76–79. doi: 10.1038/364076a0. [DOI] [PubMed] [Google Scholar]
- 254.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 255.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 256.Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 257.Mikoshiba K. The InsP3 receptor and intracellular Ca2+ signaling. Curr Opin Neurobiol. 1997;7:339–345. doi: 10.1016/s0959-4388(97)80061-x. [DOI] [PubMed] [Google Scholar]
- 258.Minagawa N, Kruglov EA, Dranoff JA, Robert ME, Gores GJ, Nathanson MH. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem. 2005;280:33637–33644. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- 259.Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, Akiba Y, Kaunitz JD, Ehrlich BE, Larusso NF, Nathanson MH. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology. 2007;133:1592–1602. doi: 10.1053/j.gastro.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mine T, Kojima I, Ogata E, Nakamura T. Comparison of effects of Hgf and Egf on cellular calcium in rat hepatocytes. Biochem Biophys Res Commun. 1991;181:1173–1180. doi: 10.1016/0006-291x(91)92062-o. [DOI] [PubMed] [Google Scholar]
- 261.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Moseley RH, Wang W, Takeda H, Lown K, Shick L, Ananthanarayanan M, Suchy FJ. Effect of endotoxin on bile acid transport in rat liver: A potential model for sepsis-associated cholestasis. Am J Physiol. 1996;271:G137–G146. doi: 10.1152/ajpgi.1996.271.1.G137. [DOI] [PubMed] [Google Scholar]
- 263.Nagata J, Guerra MT, Shugrue CA, Gomes DA, Nagata N, Nathanson MH. Lipid rafts establish calcium waves in hepatocytes. Gastroenterology. 2007;133:256–267. doi: 10.1053/j.gastro.2007.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nakamura M, Nagano H, Sakon M, Yamamoto T, Ota H, Wada H, Damdinsuren B, Noda T, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Nakamori S, Dono K, Monden M. Role of the Fas/FasL pathway in combination therapy with interferon-alpha and fluorouracil against hepatocellular carcinoma in vitro. J Hepatol. 2007;46:77–88. doi: 10.1016/j.jhep.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 265.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–566. [PubMed] [Google Scholar]
- 266.Nathanson MH, Burgstahler AD. Coordination of hormone-induced calcium signals in isolated rat hepatocyte couplets: Demonstration with confocal microscopy. Mol Biol Cell. 1992;3:113–121. doi: 10.1091/mbc.3.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nathanson MH, Burgstahler AD, Fallon MB. Multistep mechanism of polarized Ca2+ wave patterns in hepatocytes. Am J Physiol. 1994;267:G338–G349. doi: 10.1152/ajpgi.1994.267.3.G338. [DOI] [PubMed] [Google Scholar]
- 268.Nathanson MH, Burgstahler AD, Masyuk A, Larusso NF. Stimulation of ATP secretion in the liver by therapeutic bile acids. Biochem J. 2001;358:1–5. doi: 10.1042/0264-6021:3580001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nathanson MH, Burgstahler AD, Mennone A, Boyer JL. Characterization of cytosolic Ca2+ signaling in rat bile duct epithelia. Am J Physiol. 1996;271:G86–G96. doi: 10.1152/ajpgi.1996.271.1.G86. [DOI] [PubMed] [Google Scholar]
- 270.Nathanson MH, Burgstahler AD, Mennone A, Dranoff JA, Rios-Velez L. Stimulation of bile duct epithelial secretion by glybenclamide in normal and cholestatic rat liver. J Clin Invest. 1998;101:2665–2676. doi: 10.1172/JCI2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nathanson MH, Burgstahler AD, Mennone A, Fallon MB, Gonzalez CB, Saez JC. Ca2+ waves are organized among hepatocytes in the intact organ. Am J Physiol. 1995;269:G167–G171. doi: 10.1152/ajpgi.1995.269.1.G167. [DOI] [PubMed] [Google Scholar]
- 272.Nathanson MH, Fallon MB, Padfield PJ, Maranto AR. Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J Biol Chem. 1994;269:4693–4696. [PubMed] [Google Scholar]
- 273.Nathanson MH, Gautam A, Bruck R, Isales CM, Boyer JL. Effects of Ca2+ agonists on cytosolic Ca2+ in isolated hepatocytes and on bile secretion in the isolated perfused rat liver. Hepatology. 1992;15:107–116. doi: 10.1002/hep.1840150119. [DOI] [PubMed] [Google Scholar]
- 274.Nathanson MH, Gautam A, Ng OC, Bruck R, Boyer JL. Hormonal regulation of paracellular permeability in isolated rat hepatocyte couplets. Am J Physiol. 1992;262:G1079–G1086. doi: 10.1152/ajpgi.1992.262.6.G1079. [DOI] [PubMed] [Google Scholar]
- 275.Nathanson MH, Padfield PJ, O’Sullivan AJ, Burgstahler AD, Jamieson JD. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem. 1992;267:18118–18121. [PubMed] [Google Scholar]
- 276.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 277.Newton CL, Mignery GA, Sudhof TC. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J Biol Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- 278.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 279.Nicotera P, McConkey DJ, Jones DP, Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1989;86:453–457. doi: 10.1073/pnas.86.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nicou A, Serriere V, Hilly M, Prigent S, Combettes L, Guillon G, Tordjmann T. Remodelling of calcium signalling during liver regeneration in the rat. J Hepatol. 2007;46:247–256. doi: 10.1016/j.jhep.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 281.Nicou A, Serriere V, Prigent S, Boucherie S, Combettes L, Guillon G, Alonso G, Tordjmann T. Hypothalamic vasopressin release and hepatocyte Ca2+ signaling during liver regeneration: An interplay stimulating liver growth and bile flow. Hepatology. 2003;38:564A–565A. doi: 10.1096/fj.03-0082fje. [DOI] [PubMed] [Google Scholar]
- 282.Niessen H, Willecke K. Strongly decreased gap junctional permeability to inositol 1,4, 5-trisphosphate in connexin32 deficient hepatocytes. FEBS Lett. 2000;466:112–114. doi: 10.1016/s0014-5793(99)01770-6. [DOI] [PubMed] [Google Scholar]
- 283.Nutt LK, Chandra J, Pataer A, Fang B, Roth JA, Swisher SG, O’Neil RG, McConkey DJ. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J Biol Chem. 2002;277:20301–20308. doi: 10.1074/jbc.M201604200. [DOI] [PubMed] [Google Scholar]
- 284.Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, Swisher SG. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- 285.O’Brien EM, Gomes DA, Sehgal S, Nathanson MH. Hormonal regulation of nuclear permeability. J Biol Chem. 2007;282:4210–4217. doi: 10.1074/jbc.M606300200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Oakes SA, Opferman JT, Pozzan T, Korsmeyer SJ, Scorrano L. Regulation of endoplasmic reticulum Ca2+ dynamics by proapoptotic BCL-2 family members. Biochem Pharmacol. 2003;66:1335–1340. doi: 10.1016/s0006-2952(03)00482-9. [DOI] [PubMed] [Google Scholar]
- 287.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Oh JC, Jeong DL, Kim IK, Oh SH. Activation of calcium signaling by hepatitis B virus-X protein in liver cells. Exp Mol Med. 2003;35:301–309. doi: 10.1038/emm.2003.41. [DOI] [PubMed] [Google Scholar]
- 289.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 290.Oshio C, Phillips MJ. Contractility of bile canaliculi: Implications for liver function. Science. 1981;212:1041–1042. doi: 10.1126/science.7015506. [DOI] [PubMed] [Google Scholar]
- 291.Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:6198–6202. doi: 10.3748/wjg.v12.i38.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Park KS, Sin PJ, Lee DH, Cha SK, Kim MJ, Kim NH, Baik SK, Jeong SW, Kong ID. Switching-on of serotonergic calcium signaling in activated hepatic stellate cells. World J Gastroenterol. 2011;17:164–173. doi: 10.3748/wjg.v17.i2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Park SW, Zhou Y, Lee J, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107:19320–19325. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Patel S, Churchill GC, Galione A. Coordination of Ca2+ signalling by NAADP. Trends Biochem Sci. 2001;26:482–489. doi: 10.1016/s0968-0004(01)01896-5. [DOI] [PubMed] [Google Scholar]
- 295.Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, Curley SA, Ellis LM. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol. 2003;21:421–427. doi: 10.1200/JCO.2003.10.103. [DOI] [PubMed] [Google Scholar]
- 296.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: Mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 297.Peach MJ. Molecular actions of angiotensin. Biochem Pharmacol. 1981;30:2745–2751. doi: 10.1016/0006-2952(81)90410-x. [DOI] [PubMed] [Google Scholar]
- 298.Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010;1201:183–188. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- 299.Petronilli V, Cola C, Massari S, Colonna R, Bernardi P. Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J Biol Chem. 1993;268:21939–21945. [PubMed] [Google Scholar]
- 300.Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: Role of cardiolipin. J Biol Chem. 2004;279:53103–53108. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- 301.Phillips MJ, Poucell S, Oda M. Mechanisms of cholestasis. Lab Invest. 1986;54:593–608. [PubMed] [Google Scholar]
- 302.Piccoli C, Scrima R, Quarato G, D’Aprile A, Ripoli M, Lecce L, Boffoli D, Moradpour D, Capitanio N. Hepatitis C virus protein expression causes calcium-mediated mitochondrial bioenergetic dysfunction and nitro-oxidative stress. Hepatology. 2007;46:58–65. doi: 10.1002/hep.21679. [DOI] [PubMed] [Google Scholar]
- 303.Pierobon N, Renard-Rooney DC, Gaspers LD, Thomas AP. Ryanodine receptors in liver. J Biol Chem. 2006;281:34086–34095. doi: 10.1074/jbc.M607788200. [DOI] [PubMed] [Google Scholar]
- 304.Pinton P, Tsuboi T, Ainscow EK, Pozzan T, Rizzuto R, Rutter GA. Dynamics of glucose-induced membrane recruitment of protein kinase C beta II in living pancreatic islet beta-cells. J Biol Chem. 2002;277:37702–37710. doi: 10.1074/jbc.M204478200. [DOI] [PubMed] [Google Scholar]
- 305.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 306.Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, Parrington J, Galione A, Sitsapesan R. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J Biol Chem. 2010;285:35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Plevin R, Palmer S, Gardner SD, Wakelam MJ. Regulation of bombesin-stimulated inositol 1,4,5-trisphosphate generation in Swiss 3T3 fibroblasts by a guanine-nucleotide-binding protein. Biochem J. 1990;268:605–610. doi: 10.1042/bj2680605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Poenie M, Alderton J, Steinhardt R, Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986;233:886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- 309.Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N Engl J Med. 1994;330:1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 310.Pozzan T, Rizzuto R. High tide of calcium in mitochondria. Nat Cell Biol. 2000;2:E25–E27. doi: 10.1038/35000095. [DOI] [PubMed] [Google Scholar]
- 311.Pralong WF, Spat A, Wollheim CB. Dynamic pacing of cell metabolism by intracellular Ca2 +transients. J Biol Chem. 1994;269:27310–27314. [PubMed] [Google Scholar]
- 312.Pusl T, Nathanson MH. The role of inositol 1,4,5-trisphosphate receptors in the regulation of bile secretion in health and disease. Biochem Biophys Res Commun. 2004;322:1318–1325. doi: 10.1016/j.bbrc.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 313.Pusl T, Rhode F, Ott T, Drabent B, Willecke K, Beuers U. Gap junctional intercellular communication is not needed for the anticholestatic effect of tauroursodeoxycholic acid in mouse liver. J Hepatol. 2005;42:604–605. doi: 10.1016/j.jhep.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 314.Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, Hoek JB, Karpen SJ, Nathanson MH, Bennett AM. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002;277:27517–27527. doi: 10.1074/jbc.M203002200. [DOI] [PubMed] [Google Scholar]
- 315.Qin Y, Pan X, Tang TT, Zhou L, Gong XG. Anti-proliferative effects of the novel squamosamide derivative (FLZ) on HepG2 human hepatoma cells by regulating the cell cycle-related proteins are associated with decreased Ca(2+)/ROS levels. Chem Biol Interact. 2011;193:246–253. doi: 10.1016/j.cbi.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 316.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 317.Ramos-Franco J, Fill M, Mignery GA. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J. 1998;75:834–839. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Rasmussen CD, Means AR. The presence of parvalbumin in a nonmuscle cell-line attenuates progression through mitosis. Mol Endocrinol. 1989;3:588–596. doi: 10.1210/mend-3-3-588. [DOI] [PubMed] [Google Scholar]
- 319.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 320.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 321.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 322.Reinehr RM, Kubitz R, Peters-Regehr T, Bode JG, Haussinger D. Activation of rat hepatic stellate cells in culture is associated with increased sensitivity to endothelin 1. Hepatology. 1998;28:1566–1577. doi: 10.1002/hep.510280617. [DOI] [PubMed] [Google Scholar]
- 323.Rhee SG, Choi KD. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992;267:12393–12396. [PubMed] [Google Scholar]
- 324.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 325.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 326.Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Robb-Gaspers LD, Rutter GA, Burnett P, Hajnoczky G, Denton RM, Thomas AP. Coupling between cytosolic and mitochondrial calcium oscillations: Role in the regulation of hepatic metabolism. Biochim Biophys Acta. 1998;1366:17–32. doi: 10.1016/s0005-2728(98)00118-2. [DOI] [PubMed] [Google Scholar]
- 328.Robb-Gaspers LD, Thomas AP. Coordination of Ca2+ signaling by intercellular propagation of Ca2+ waves in the intact liver. J Biol Chem. 1995;270:8102–8107. doi: 10.1074/jbc.270.14.8102. [DOI] [PubMed] [Google Scholar]
- 329.Roberts SK, Ludwig J, LaRusso NF. The pathobiology of biliary epithelia. Gastroenterology. 1997;112:269–279. doi: 10.1016/s0016-5085(97)70244-0. [DOI] [PubMed] [Google Scholar]
- 330.Rockey DC. Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis. 2001;21:337–349. doi: 10.1055/s-2001-17551. [DOI] [PubMed] [Google Scholar]
- 331.Rodrigues MA, Gomes DA, Andrade VA, Leite MF, Nathanson MH. Insulin induces calcium signals in the nucleus of rat hepatocytes. Hepatology. 2008;48:1621–1631. doi: 10.1002/hep.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, Cheng YC, Bennett AM, Nathanson MH. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem. 2007;282:17061–17068. doi: 10.1074/jbc.M700490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Roma MG, Crocenzi FA, Mottino AD. Dynamic localization of hepatocellular transporters in health and disease. World J Gastroenterol. 2008;14:6786–6801. doi: 10.3748/wjg.14.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Roman RM, Bodily KO, Wang Y, Raymond JR, Fitz JG. Activation of protein kinase C alpha couples cell volume to membrane Cl- permeability in HTC hepatoma and Mz-ChA-1 cholangiocarcinoma cells. Hepatology. 1998;28:1073–1080. doi: 10.1002/hep.510280423. [DOI] [PubMed] [Google Scholar]
- 335.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 336.Rooney TA, Renard DC, Sass EJ, Thomas AP. Oscillatory cytosolic calcium waves independent of stimulated inositol 1,4,5-trisphosphate formation in hepatocytes. J Biol Chem. 1991;266:12272–12282. [PubMed] [Google Scholar]
- 337.Rooney TA, Sass EJ, Thomas AP. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989;264:17131–17141. [PubMed] [Google Scholar]
- 338.Rooney TA, Sass EJ, Thomas AP. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J Biol Chem. 1990;265:10792–10796. [PubMed] [Google Scholar]
- 339.Rottingen J, Iversen JG. Ruled by waves? Intracellular and intercellular calcium signalling. Acta Physiol Scand. 2000;169:203–219. doi: 10.1046/j.1365-201x.2000.00732.x. [DOI] [PubMed] [Google Scholar]
- 340.Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, Cheng X, Churchill GC, Zhu MX, Platt FM, Wessel GM, Parrington J, Galione A. Purified TPC isoforms form NAADP receptors with distinct roles for Ca(2+) signaling and endolysosomal trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rudnick DA, Perlmutter DH, Muglia LJ. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proc Natl Acad Sci U S A. 2001;98:8885–8890. doi: 10.1073/pnas.151217998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Rusinko N, Lee HC. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989;264:11725–11731. [PubMed] [Google Scholar]
- 343.Rutter GA, Rizzuto R. Regulation of mitochondrial metabolism by ER Ca2+ release: An intimate connection. Trends Biochem Sci. 2000;25:215–221. doi: 10.1016/s0968-0004(00)01585-1. [DOI] [PubMed] [Google Scholar]
- 344.Saez JC, Connor JA, Spray DC, Bennett MV. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, Kawata S, Imai Y, Iijima S, Monden M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;95:2581. doi: 10.1002/cncr.10246. [DOI] [PubMed] [Google Scholar]
- 346.Sanderson MJ, Charles AC, Boitano S, Dirksen ER. Mechanisms and function of intercellular calcium signaling. Mol Cell Endocrinol. 1994;98:173–187. doi: 10.1016/0303-7207(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 347.Santella L, Carafoli E. Calcium signaling in the cell nucleus. FASEB J. 1997;11:1091–1109. [PubMed] [Google Scholar]
- 348.Satoh T, Ross CA, Villa A, Supattapone S, Pozzan T, Snyder SH, Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: Quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990;111:615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Scarpa A, Azzone GF. The mechanism of ion translocation in mito-chondria. 4. Coupling of K+ efflux with Ca2+ uptake. Eur J Biochem. 1970;12:328–335. doi: 10.1111/j.1432-1033.1970.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 350.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 351.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci U S A. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Servillo G, Della Fazia MA, Sassone-Corsi P. Coupling cAMP signaling to transcription in the liver: Pivotal role of CREB and CREM. Exp Cell Res. 2002;275:143–154. doi: 10.1006/excr.2002.5491. [DOI] [PubMed] [Google Scholar]
- 353.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 354.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Shirakawa H, Miyazaki S. Spatiotemporal analysis of calcium dynamics in the nucleus of hamster oocytes. J Physiol. 1996;494 (Pt 1):29–40. doi: 10.1113/jphysiol.1996.sp021473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Shoshan-Barmatz V. High affinity ryanodine binding sites in rat liver endoplasmic reticulum. FEBS Lett. 1990;263:317–320. doi: 10.1016/0014-5793(90)81403-b. [DOI] [PubMed] [Google Scholar]
- 357.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 358.Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Soliman EM, Rodrigues MA, Gomes DA, Sheung N, Yu J, Amaya MJ, Nathanson MH, Dranoff JA. Intracellular calcium signals regulate growth of hepatic stellate cells via specific effects on cell cycle progression. Cell Calcium. 2009;45:284–292. doi: 10.1016/j.ceca.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Spirli C, Nathanson MH, Fiorotto R, Duner E, Denson LA, Sanz JM, Di Virgilio F, Okolicsanyi L, Casagrande F, Strazzabosco M. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156–169. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 361.Staddon JM, Hansford RG. Evidence indicating that the glucagon-induced increase in cytoplasmic free Ca2+ concentration in hepatocytes is mediated by an increase in cyclic AMP concentration. Eur J Biochem. 1989;179:47–52. doi: 10.1111/j.1432-1033.1989.tb14519.x. [DOI] [PubMed] [Google Scholar]
- 362.Stehno-Bittel L, Luckhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 363.Steiling H, Wustefeld T, Bugnon P, Brauchle M, Fassler R, Teupser D, Thiery J, Gordon JI, Trautwein C, Werner S. Fibroblast growth factor receptor signalling is crucial for liver homeostasis and regeneration. Oncogene. 2003;22:4380–4388. doi: 10.1038/sj.onc.1206499. [DOI] [PubMed] [Google Scholar]
- 364.Steinhardt RA, Alderton J. Intracellular free calcium rise triggers nuclear-envelope breakdown in the sea-urchin embryo. Nature. 1988;332:364–366. doi: 10.1038/332364a0. [DOI] [PubMed] [Google Scholar]
- 365.Stoffler D, Goldie KN, Feja B, Aebi U. Calcium-mediated structural changes of native nuclear pore complexes monitored by time-lapse atomic force microscopy. J Mol Biol. 1999;287:741–752. doi: 10.1006/jmbi.1999.2637. [DOI] [PubMed] [Google Scholar]
- 366.Strambio-De-Castillia C, Niepel M, Rout MP. The nuclear pore complex: Bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol. 2010;11:490–501. doi: 10.1038/nrm2928. [DOI] [PubMed] [Google Scholar]
- 367.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a non-mitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 368.Stumpel F, Ott T, Willecke K, Jungermann K. Connexin 32 gap junctions enhance stimulation of glucose output by glucagon and noradrenaline in mouse liver. Hepatology. 1998;28:1616–1620. doi: 10.1002/hep.510280622. [DOI] [PubMed] [Google Scholar]
- 369.Sudhof TC, Newton CL, Archer BT, III, Ushkaryov YA, Mignery GA. Structure of a novel InsP3 receptor. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sudhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Swillens S, DuPont G, Combettes L, Champeil P. From calcium blips to calcium puffs: Theoretical analysis of the requirements for interchannel communication. Proc Natl Acad Sci U S A. 1999;96:13750–13755. doi: 10.1073/pnas.96.24.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Mol Cell Biol. 1999;19:6003–6011. doi: 10.1128/mcb.19.9.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Tanaka Y, Hayashi N, Kaneko A, Ito T, Miyoshi E, Sasaki Y, Fusamoto H, Kamada T. Epidermal growth-factor induces dose-dependent calcium oscillations in single fura-2 loaded hepatocytes. Hepatology. 1992;16:479–486. doi: 10.1002/hep.1840160229. [DOI] [PubMed] [Google Scholar]
- 374.Tang J, Wu YM, Zhao P, Jiang JL, Chen ZN. beta ig-h3 Interacts with alpha 3 beta 1 integrin to promote adhesion and migration of human hepatoma cells. Exp Biol Med. 2009;234:35–39. doi: 10.3181/0806-RM-187. [DOI] [PubMed] [Google Scholar]
- 375.Tardif KD, Mori K, Kaufman RJ, Siddiqui A. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J Biol Chem. 2004;279:17158–17164. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- 376.Tardif KD, Waris G, Siddiqui A. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 2005;13:159–163. doi: 10.1016/j.tim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 377.Taub R. Liver regeneration: From myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 378.Thapa N, Lee BH, Kim IS. TGFBIp/beta ig-h3 protein: A versatile matrix molecule induced by TGF-beta. Int J Biochem Cell Biol. 2007;39:2183–2194. doi: 10.1016/j.biocel.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 379.Thomas AP, Bird GS, Hajnoczky G, Robb-Gaspers LD, Putney JW., Jr Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- 380.Thomas AP, Martin-Requero A, Williamson JR. Interactions between insulin and alpha 1-adrenergic agents in the regulation of glycogen metabolism in isolated hepatocytes. J Biol Chem. 1985;260:5963–5973. [PubMed] [Google Scholar]
- 381.Thomas AP, Renard DC, Rooney TA. Spatial and temporal organization of calcium signalling in hepatocytes. Cell Calcium. 1991;12:111–126. doi: 10.1016/0143-4160(91)90013-5. [DOI] [PubMed] [Google Scholar]
- 382.Thomas AP, Robb-Gaspers LD, Rooney TA, Hajnoczky G, Renard-Rooney DC, Lin C. Spatial organization of oscillating calcium signals in liver. Biochem Soc Trans. 1995;23:642–648. doi: 10.1042/bst0230642. [DOI] [PubMed] [Google Scholar]
- 383.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 384.Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 2003;22:3825–3832. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tordjmann T, Berthon B, Claret M, Combettes L. Coordinated intercellular calcium waves induced by noradrenaline in rat hepatocytes: Dual control by gap junction permeability and agonist. EMBO J. 1997;16:5398–5407. doi: 10.1093/emboj/16.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Tordjmann T, Berthon B, Combettes L, Claret M. The location of hepatocytes in the rat liver acinus determines their sensitivity to calcium-mobilizing hormones. Gastroenterology. 1996;111:1343–1352. doi: 10.1053/gast.1996.v111.pm8898649. [DOI] [PubMed] [Google Scholar]
- 387.Tordjmann T, Berthon B, Jacquemin E, Clair C, Stelly N, Guillon G, Claret M, Combettes L. Receptor-oriented intercellular calcium waves evoked by vasopressin in rat hepatocytes. EMBO J. 1998;17:4695–4703. doi: 10.1093/emboj/17.16.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 389.Trusolino L, Bertotti A, Comoglio PM. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 390.Twigg J, Patel R, Whitaker M. Translational Control of InsP3-induced chromatin condensation during the early cell-cyc les of sea-urchin embryos. Nature. 1988;332:366–369. doi: 10.1038/332366a0. [DOI] [PubMed] [Google Scholar]
- 391.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 393.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Visnjic D, Banfic H. Nuclear phospholipid signaling: Phosphatidylinositol-specific phospholipase C and phosphoinositide 3-kinase. Pflugers Arch. 2007;455:19–30. doi: 10.1007/s00424-007-0288-1. [DOI] [PubMed] [Google Scholar]
- 395.Wakelam MJ, Murphy GJ, Hruby VJ, Houslay MD. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986;323:68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- 396.Wakui M, Potter BV, Petersen OH. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989;339:317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- 397.Walker SA, Kupzig S, Bouyoucef D, Davies LC, Tsuboi T, Bivona TG, Cozier GE, Lockyer PJ, Buckler A, Rutter GA, Allen MJ, Philips MR, Cullen PJ. Identification of a Ras GTPase-activating protein regulated by receptor-mediated Ca2+ oscillations. EMBO J. 2004;23:1749–1760. doi: 10.1038/sj.emboj.7600197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Watanabe N, Tsukada N, Smith CR, Edwards V, Phillips MJ. Permeabilized hepatocyte couplets. Adenosine triphosphate-dependent bile canalicular contractions and a circumferential pericanalicular microfilament belt demonstrated. Lab Invest. 1991;65:203–213. [PubMed] [Google Scholar]
- 399.Watanabe N, Tsukada N, Smith CR, Phillips MJ. Motility of bile canaliculi in the living animal: Implications for bile flow. J Cell Biol. 1991;113:1069–1080. doi: 10.1083/jcb.113.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Watanabe S, Smith CR, Phillips MJ. Coordination of the contractile activity of bile canaliculi. Evidence from calcium microinjection of triplet hepatocytes. Lab Invest. 1985;53:275–279. [PubMed] [Google Scholar]
- 401.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Wei Y, Wang D, Gentile CL, Pagliassotti MJ. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem. 2009;331:31–40. doi: 10.1007/s11010-009-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006a;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Whitaker M. Calcium microdomains and cell cycle control. Cell Calcium. 2006b;40:585–592. doi: 10.1016/j.ceca.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-X-L modulation of the InsP(3)R. Nat Cell Biol. 2005;7:1021–U135. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 407.Williams TF, Exton JH, Friedmann N, Park CR. Effects of insulin and adenosine 3′,5′-monophosphate on K+ flux and glucose output in perfused rat liver. Am J Physiol. 1971;221:1645–1651. doi: 10.1152/ajplegacy.1971.221.6.1645. [DOI] [PubMed] [Google Scholar]
- 408.Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Taurour-sodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- 409.Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 410.Wojcikiewicz RJ, Ernst SA, Yule DI. Secretagogues cause ubiquitination and down-regulation of inositol 1, 4,5-trisphosphate receptors in rat pancreatic acinar cells. Gastroenterology. 1999;116:1194–1201. doi: 10.1016/s0016-5085(99)70023-5. [DOI] [PubMed] [Google Scholar]
- 411.Woo K, Sathe M, Kresge C, Esser V, Ueno Y, Venter J, Glaser SS, Alpini G, Feranchak AP. Adenosine triphosphate release and purinergic (P2) receptor-mediated secretion in small and large mouse cholangiocytes. Hepatology. 2010;52:1819–1828. doi: 10.1002/hep.23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Woods NM, Cuthbertson KS, Cobbold PH. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986;319:600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- 413.Woods NM, Cuthbertson KS, Cobbold PH. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987;8:79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- 414.Wu DQ, Lee CH, Rhee SG, Simon MI. Activation of phospholipase C by the alpha subunits of the Gq and G11 proteins in transfected Cos-7 cells. J Biol Chem. 1992;267:1811–1817. [PubMed] [Google Scholar]
- 415.Yamaguchi Y, Dalle-Molle E, Hardison WG. Vasopressin and A23187 stimulate phosphorylation of myosin light chain-1 in isolated rat hepatocytes. Am J Physiol. 1991;261:G312–G319. doi: 10.1152/ajpgi.1991.261.2.G312. [DOI] [PubMed] [Google Scholar]
- 416.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Yamamoto-Hino M, Miyawaki A, Segawa A, Adachi E, Yamashina S, Fujimoto T, Sugiyama T, Furuichi T, Hasegawa M, Mikoshiba K. Apical vesicles bearing inositol 1,4,5-trisphosphate receptors in the Ca2+ initiation site of ductal epithelium of submandibular gland. J Cell Biol. 1998;141:135–142. doi: 10.1083/jcb.141.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Yamasaki M, Masgrau R, Morgan AJ, Churchill GC, Patel S, Ashcroft SJ, Galione A. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 419.Yang B, Bouchard MJ. The hepatitis B virus X protein elevates cytosolic calcium signals by modulating mitochondrial calcium uptake. J Virol. 2012;86:313–327. doi: 10.1128/JVI.06442-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Yang S, Huang XY. Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem. 2005;280:27130–27137. doi: 10.1074/jbc.M501625200. [DOI] [PubMed] [Google Scholar]
- 421.Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol. 1995;482 (Pt 3):533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Yin H, Xie F, Zhang J, Yang Y, Deng B, Sun J, Wang Q, Qu X, Mao H. Combination of interferon-alpha and 5-fluorouracil induces apoptosis through mitochondrial pathway in hepatocellular carcinoma in vitro. Cancer Lett. 2011;306:34–42. doi: 10.1016/j.canlet.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 423.Zhang Y, Xue R, Zhang Z, Yang X, Shi H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis. 2012;11:1. doi: 10.1186/1476-511X-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rotzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Zoulim F, Saputelli J, Seeger C. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J Virol. 1994;68:2026–2030. doi: 10.1128/jvi.68.3.2026-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]