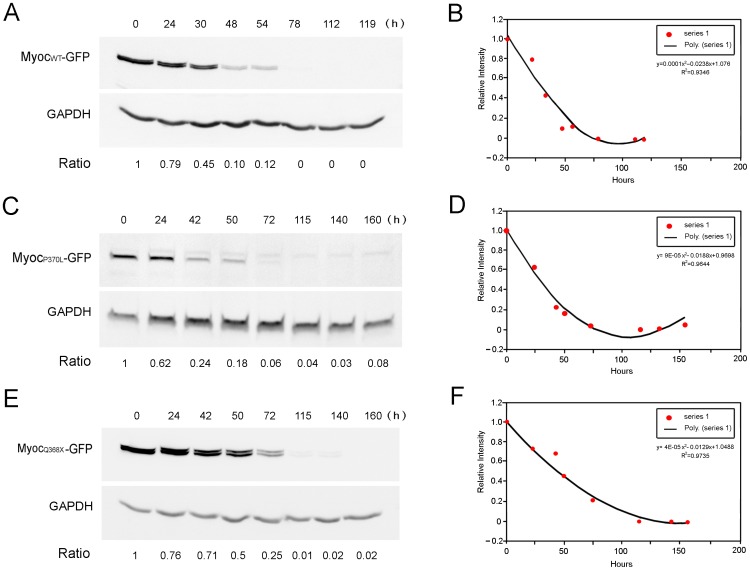
Figure 5. Turnover of overexpressed myocilin (wild-type or mutant)-GFP fusion proteins.
Tet-on inducible RGC5 cells were treated with Dox (1 µg/ml) for 24 h to express a moderate level of wild-type (MyocWT, A), mutant P370L (MyocP370L, C) or Q368X (MyocQ368X, E) myocilin-GFP fusion protein. After washing, cells were incubated in growth medium containing 1% fetal bovine serum (without Dox) and were harvested at various time points. Monesin (2 µM) was added to the wild-type myocilin-expressing cells to block the myocilin secretion. The levels of fusion proteins were measured by Western blotting. The relative intensity of the induced wild-type (B), P370L (D), and Q368X (F) myocilin-GFP to that of GAPDH was plotted against time to estimate the half-life. The half-life of wild-type-, P370L-, and Q368X-myocilin-GFP was approximately 24, 42, and 50 h, respectively. The turnover rate of overexpressed wild-type myocilin and mutants was much slower than that of the endogenous myocilin, suggesting that the protein processing was altered upon myocilin overexpression or mutation. All experiments were repeated at least 3 times, yielding similar results.