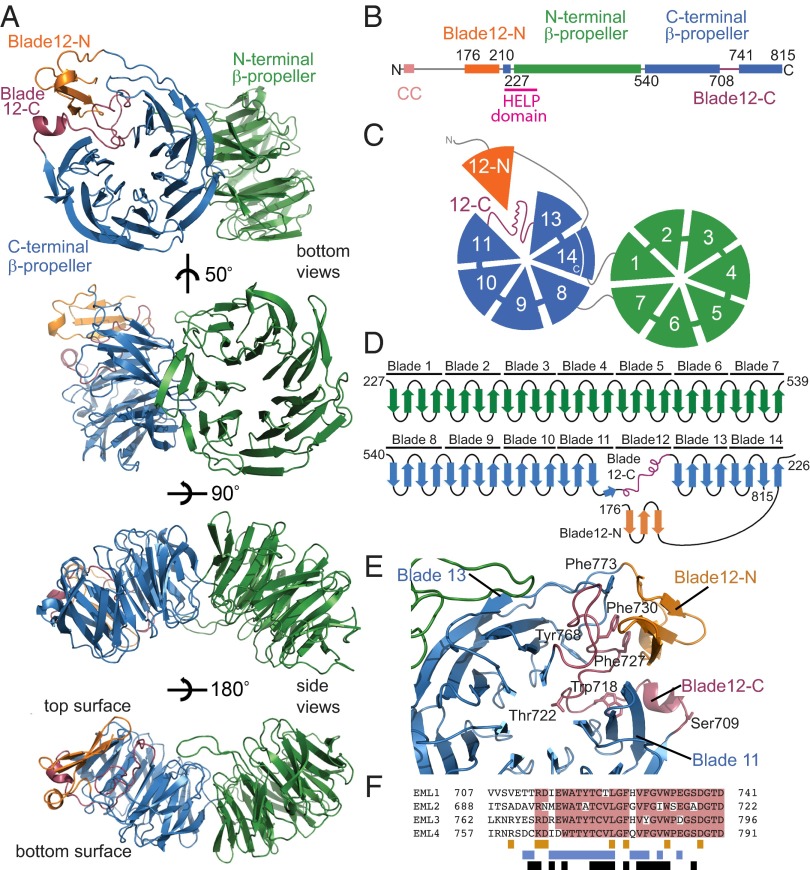
Fig. 1.
Architecture of the EML1 TAPE domain. Throughout the figure, the subdomains are colored by the following scheme: orange (blade12-N), green (N-terminal propeller), blue (C-terminal propeller), and purple (blade12-C). (A) The structure of the EML1 TAPE domain is shown as cartoon representations of bottom and side views. (B) Linear representation of the EML1 protein including residue numbering indicating domain boundaries. (C) Cartoon representation showing the bottom face of the TAPE domain with numbered blades. (D) Topology diagram showing connectivity of the secondary structure elements. (E) View of the C-terminal β-propeller from above its central channel showing how blade 12 is formed from blade12-N and blade12-C. Key residues are shown as stick representations. (F) Sequence alignment indicating sequence conservation among human EML proteins (pink) in the region corresponding to blade12-C. The colored bars under the alignment indicate residues that make contacts with blades 11 and 13 (blue), with blade12-N (orange), and with those that are buried (black).