Fig. 5.
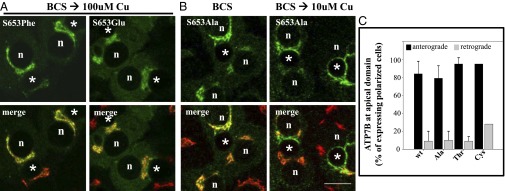
ATP7BS653F and ATP7BS653E mutant proteins are retained in the TGN in 100-μM copper, whereas ATP7BS653A mutant protein exhibits wild-type trafficking. WIF-B cells were infected with the indicated ATP7B adenovirus, cultured, and processed as described in Methods. Single confocal sections are shown. (A) Substitution of S653 with either phenylalanine (F, Phe) or glutamate (E, Glu) blocked TGN-exit of ATP7B in 100 μM copper. A cytoplasmic green haze found in ATP7BS653E-expressing cells may reflect its degradation (Fig. S3A). (B) In contrast, ATP7BS653A redistributed from the TGN (B, Left) to vesicles and the apical membrane when copper levels were elevated (B, Right). n, nucleus; *, apical space. (Scale bar,10 μm.) (C) Quantitative evidence that ATP7BS653A, ATP7BS653T and ATP7BS653C substitutions show wild-type trafficking in polar hepatic cells. WIF-B cells infected with adenoviruses encoding wtATP7B and three 653 substitutions were cultured, processed, and quantitatively analyzed for trafficking as described in Fig. 3. Like wtATP7B, ATP7BS653A, ATP7BS653T, and ATP7BS653C variants exited the TGN (anterograde) in 10-μM copper and returned (retrograde) when copper levels were lowered. The number of polar cells evaluated for anterograde/retrograde trafficking, respectively, were Ala: 108/115; Thr: 98/142; and Cys: 158/77.
