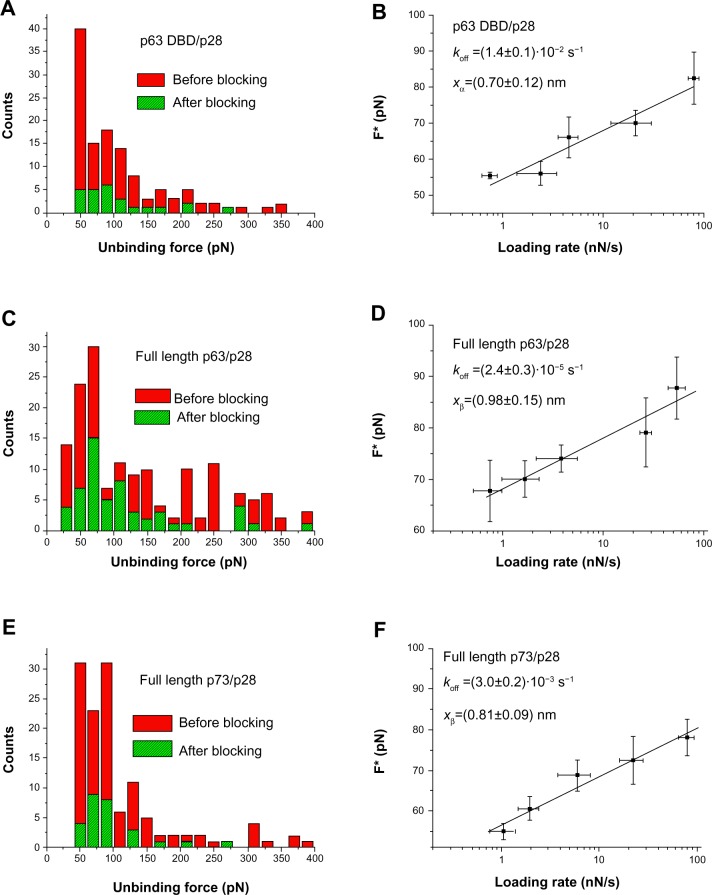
Figure 4.
AFS analysis of p28 binding.
Notes: (A) Histogram of the unbinding force for the p63 DBD/p28 complex recorded at a loading rate of 7 nN/s before (red bars) and after (green bars) blocking the p63 DBD monolayer. (B) Plot of the most probable unbinding forces versus the natural logarithm of the different loading rates of the p63 DBD/p28 interaction. The line represents the fit of the experimental data by the Bell–Evans model (Equation 1); the goodness of the fit being assessed by both the linear coefficient correlation (ρ=0.96) and the reduced chi-square (χ2=1.4). The kinetic parameters obtained are reported in the inset with the relative uncertainties. (C) Histogram of the unbinding force distribution for the full-length p63/p28 complex acquired at the loading rate 7 nN/s before (red bars) and after (green bars) blocking the p63 monolayer. (D) Plot of the most probable unbinding forces versus the natural logarithm of the different loading rates of the full-length p63/p28 interaction. The line represents the fit of the experimental data by the Bell–Evans model (Equation 1); the goodness of the fit being assessed by both the linear coefficient correlation (ρ=0.97) and the reduced chi-square (χ2=1.3). Kinetic values appear in the inset with their relative uncertainties. (E) Histogram of the unbinding force distribution for the full-length p73/p28 complex acquired at the loading rate 7 nN/s before (red bars) and after (green bars) blocking the p73 monolayer. (F) Plot of the most probable unbinding forces versus the natural logarithm of the different loading rates of the full-length p73/p28 interaction. The line represents the ft of the experimental data by the Bell–Evans model (Equation 1); the goodness of the fit being assessed by both the linear coefficient correlation (ρ=0.98) and the reduced chi-square (χ2=1.2). The kinetic parameters obtained are reported in the inset with the relative uncertainties.
Abbreviations: DBD, DNA-binding domain; AFS, atomic force spectroscopy.