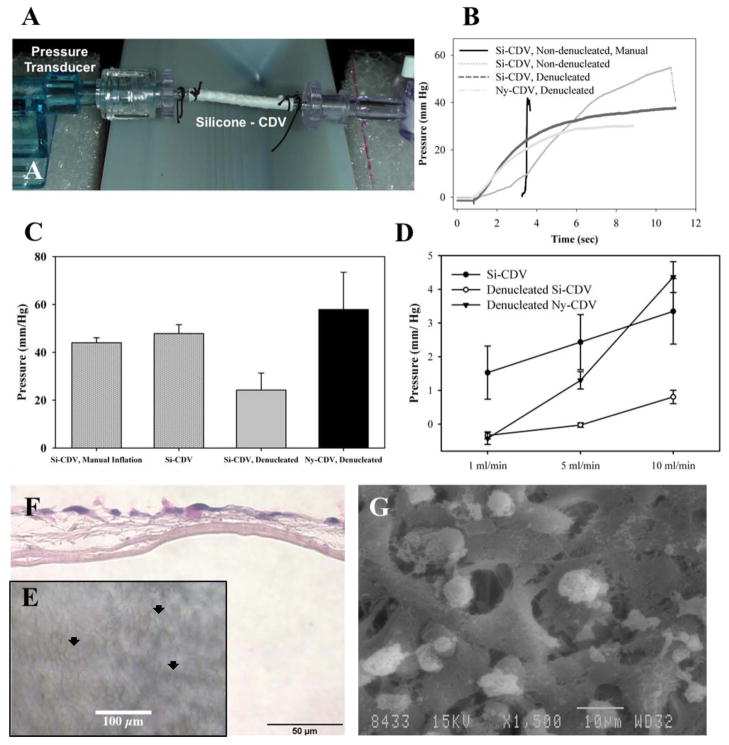
Figure 3.
Characterization of CDVs. A) Flow testing setup with in-line pressure transducer on one end, showing an Si-CDV mounted between two barb connectors and an valve at the other end. The tubing is connected to a continuous flow pump. B) Comparison of steady state intraluminal pressure development during manual inflation and continuous flow inflation with a syringe pump. The CDV devices were evaluated both dry and after denucleation using ethanol C) CDV devices were subjected to constant low flow infusion and steady state intraluminal pressure measured.. D) Peak pressure at failure (burst or major fluid leakage) of CDVs when inflated at constant flow rate against a blocked outflow. E) Biocompatibility of Si-CDV was evaluated in vitro using human HaCaT epithelial cells seeded onto CDVs in vitro. The optical characteristics of the Si-CDV permit visualization of cells on the surface of the devices using phase contrast imaging. Arrowheads identify examples of cellular coverage. F) H&E stained paraffin processed section of the in-vitro cell layer showing growth of cells but lack of penetration through inner low porosity electrospun layer. G) SEM image of cells growing and attaching to larger outer fibers of a Si-CDV in-vitro.