Abstract
Purpose
Buparlisib, an oral reversible inhibitor of all class I phosphoinositide-3-kinases, has shown antitumoral activity against estrogen receptor (ER)-positive breast cancer cell lines and xenografts, alone and with endocrine therapy. This phase Ib study evaluated buparlisib plus letrozole's safety, tolerability, and preliminary activity in patients with metastatic ER-positive breast cancer refractory to endocrine therapy.
Patients and Methods
Patients received letrozole and buparlisib in two different administration schedules. Outcomes were assessed by standard solid-tumor phase I methods. [18F]fluorodeoxyglucose–positron emission tomography/computed tomography ([18F]FDG-PET/CT) scans were done at baseline and 2 weeks after treatment initiation. Tumor blocks were collected for phosphoinositide-3-kinase pathway mutation analysis.
Results
Fifty-one patients were allocated sequentially to continuous or intermittent (five on/two off days) buparlisib administration on an every-4-week schedule. Buparlisib's maximum-tolerated dose (MTD) was 100 mg/d. Common drug-related adverse events included ≤ grade 2 hyperglycemia, nausea, fatigue, transaminitis, and mood disorders. The clinical benefit rate (lack of progression ≥ 6 months) among all patients treated at the MTD was 31%, including two objective responses in the continuous dose arm. Of seven patients remaining on treatment ≥ 12 months, three had tumors with PIK3CA hot-spot mutation. Patients exhibiting metabolic disease progression by [18F]FDG-PET/CT scan at 2 weeks progressed rapidly on therapy.
Conclusion
The letrozole and buparlisib combination was safe, with reversible toxicities regardless of schedule administration. Clinical activity was observed independent of PIK3CA mutation status. No metabolic response by [18F]FDG-PET/CT scan at 2 weeks was associated with rapid disease progression. Phase III trials of buparlisib and endocrine therapy in patients with ER-positive breast cancer are ongoing.
INTRODUCTION
The phosphoinositide-3-kinase (PI3K) pathway is the most frequently altered pathway in cancer, with mutation and/or amplification of the genes encoding the PI3K catalytic subunits p110α (PIK3CA; most common one), p110β (PIK3CB), and the PI3K regulatory subunit p85α (PIK3R1). PIK3CA mutations induce a transformed phenotype including growth factor- and anchorage-independent growth, resistance to anoikis, and drug resistance.1–4 Approximately 40% of estrogen receptor (ER) –positive breast cancers harbor PIK3CA mutations.5–7
We and others have shown preclinically that activation of the PI3K signaling pathway promotes resistance to endocrine therapy.8 PI3K signaling has been shown to promote estrogen-independent growth of ER-positive breast cancer cells9,10; however, this growth is inhibited by the addition of PI3K-inhibitors to antiestrogens.11 Additionally, inhibition of PI3K prevents the emergence of hormone-independent cells, which suggests that early intervention with antiestrogens and PI3K-inhibitors could limit escape from endocrine therapy.
Drugs targeting multiple levels of the PI3K network have been developed.12,13 Buparlisib (BKM120; Novartis Pharma AG, Basel, Switzerland)14 is an oral pyrimidine-derived reversible pan-PI3K inhibitor, with specific and potent activity against mutant PI3Kα, as well as wild-type PI3Kα, β, γ, and δ class I isoforms, but no inhibitory activity against the class III PI3K or mammalian target of rapamycin (mTOR). A phase I study of single agent buparlisib demonstrated that at the maximum-tolerated dose (MTD) of 100 mg/d, buparlisib is safe and well tolerated, exhibiting a favorable pharmacokinetic profile, with clear evidence of target inhibition and preliminary antitumor activity.15
The primary objective of our phase Ib trial was to determine the safety and tolerability of oral letrozole, an aromatase inhibitor, in combination with buparlisib in patients with ER-positive metastatic breast cancer refractory to endocrine therapies. Secondary objectives included antitumor activity and pharmacodynamic assessment of tumor metabolic response by [18F]fluorodeoxyglucose–positron emission tomography/computed tomography ([18F]FDG-PET/CT) scan. Clinical outcome was correlated with presence of PIK3CA mutations in tumor specimens.
PATIENTS AND METHODS
Patient Population
Postmenopausal patients had histologically confirmed ER-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer refractory to at least one line of endocrine therapy in the metastatic setting, or diagnosed with metastatic breast cancer during or within 1 year of adjuvant endocrine therapy; evaluable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST); age ≥ 18 years; life expectancy ≥ 6 months; Eastern Cooperative Oncology Group performance status ≤ 1; adequate bone marrow, hepatic, and renal function; and fasting plasma glucose levels ≤ 140 mg/dL (7.8 mmol/L). A tumor specimen (primary or metastatic) from archival material or fresh biopsy was required. Key exclusion criteria were CYP3A4 modifier drug treatment ≤ 2 weeks before starting buparlisib, clinically manifest diabetes mellitus, clinically documented depression or anxiety on the Patient Health Questionnaire–9 (PHQ-9) and Generalized Anxiety Disorder Screener–7 (GAD-7) mood scales, and previous treatment with PI3K-inhibitors.
Primary endocrine therapy resistance was defined as relapses during or within 6 months of stopping adjuvant endocrine treatment, or progression within 6 months of starting endocrine treatment in the metastatic setting. Secondary resistance was defined as relapses > 6 months after stopping adjuvant endocrine therapy or responses for ≥ 6 months to endocrine therapy in the metastatic setting.
Approval was obtained from the ethics committees (institutional review board no. 101057, Vanderbilt University) at the participating institutions and regulatory authorities. All patients gave informed consent. The study followed the Declaration of Helsinki and Good Clinical Practice guidelines.
Study Design
This phase Ib, multicenter, open-label study enrolled subjects in a standard 3 + 3 dose de-escalation design. All patients received letrozole 2.5 mg/d, and buparlisib was initiated at 100 mg/d (MTD of the single agent phase I trial15), on a 28-day cycle. In case of adverse events requiring dose adjustments, buparlisib doses were reduced to 80 mg/d and subsequently to 60 mg/d. Intrapatient dose reductions were allowed after the initial 4 weeks of treatment. Patients were treated until disease progression, unacceptable toxicity, or consent withdrawal.
Dose-limiting toxicities (DLTs) were defined as Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 grade ≥ 3 toxicities. Exceptions were grade ≥ 2 pancreatitis, ≥ 1 grade level increase in neurotoxicity, grade ≥ 2 phototoxicity or skin rash necessitating treatment interruption for > 21 consecutive days, non-CTCAE grade 2 hyperglycemia not resolved to grade 0 within 14 consecutive days of initiation of oral antidiabetes medications, non-CTCAE grade ≥ 3 hyperglycemia, grade 2 mood alterations not resolved to grade 1 within 14 days despite medical treatment (grade 2 anxiety was considered a DLT only if worsened from baseline), and grade ≥ 3 mood alterations. CTCAE grade ≥ 3 anemia was not considered a DLT unless judged to be a hemolytic process secondary to study drug. CTCAE grade ≥ 3 lymphopenia was not considered a DLT unless clinically significant.
The MTD was defined as the highest dose of buparlisib in combination with letrozole not causing DLT in > 33% of patients in the first treatment cycle. Twenty or more evaluable patients had to be treated before declaration of an MTD, with ≥ six evaluable patients treated at the MTD for one cycle. Criteria for evaluation were ≥ 21 days on buparlisib treatment in cycle 1 or early discontinuation because of a DLT. The recommended phase II dose (in each arm) was defined as the highest dose at or below the MTD at which ≥ 75% of the patients could tolerate therapy for a minimum of 8 weeks without development of CTCAE ≥ 2 hyperglycemia for more than 14 consecutive days despite initiation of oral antidiabetes medications; and CTCAE ≥ 3 rash, CTCAE ≥ 2 nausea, vomiting or diarrhea, and CTCAE ≥ 2 rash, all for more than 14 consecutive days of initiation of optimal medical treatment.
Buparlisib was administered once daily in a continuous schedule. Once 20 patients were accrued, the protocol was amended to add a second expansion arm, in which buparlisib was administered in an intermittent schedule (5 days of 7). Cross-over between arms was not allowed. Patients accrued to the second arm of the study were treated in three different institutions.
Safety and Radiographic Assessments
Clinical and laboratory assessments were conducted at baseline and weekly during cycle 1, on days 1 and 15 of cycle 2, and on day 1 of subsequent cycles. Safety assessments included serial ECGs, fasting plasma glucose, and neuropsychiatric assessments (self-rating mood questionnaires GAD-7 [anxiety] and PHQ-9 [depression]). Adverse events were graded by using the CTCAE version 4.0. Radiographic responses were assessed every 2 months by using RECIST version 1.1.
[18F]FDG-PET/CT Scan Assessments
Whole-body [18F]FDG-PET/CT scans were performed at Vanderbilt University with a 60-minute FDG-uptake period at three time points: baseline, on day 15 of treatment, and after 2 months of treatment, on patients in the continuous treatment arm. PET/CT scanner qualification, central quality assurance, and image analysis were performed by the imaging core laboratory at the Dana-Farber Cancer Institute.
Metabolic response was assessed on the basis of the post-treatment change relative to baseline in the sum of the maximum standardized uptake value of up to five lesions for each patient (partial response [PR] ≤ 25% reduction, stable disease [SD] < 25% reduction or increase, progressive disease > 25% increase16; Appendix, online only). The agreement between the cycle 1 and cycle 2 metabolic response assessments was evaluated by using Kendall's W test.
Mutation Analysis
DNA was extracted by using DNEasy (Qiagen, CA) or QiaAMP DNA (Qiagen) tissue kits from formalin-fixed paraffin-embedded archival tumor sections or fresh biopsies of metastases, respectively. Tumor cellularity was assessed by an expert breast pathologist (M.G.K. or M.E.S.), and specimens with > 20% tumor nuclei were included and considered evaluable. In paucicellular samples that did not exceed 20%, multiple sections were macro-dissected to achieve 20% tumor cellularity. DNA was subjected to Snapshot17 analysis (Appendix, online only) of 18 mutations in PIK3CA, PTEN, and AKT1, including the common hot-spot mutations in exons 9 and 20 of PIK3CA (Table 1).
Table 1.
Snapshot Analysis of 18 Mutations in PIK3CA, PTEN, and AKT1
| Position | AA Mutant | Nucleotide Mutant |
|---|---|---|
| PIK3CA | ||
| H1047 | p.H1047R | c.3140A>G |
| p.H1047L | c.3140A>T | |
| E542 | p.E542K | c.1624G>A |
| E545 | p.E545K | c.1633G>A |
| p.E545Q | c.1633G>C | |
| p.E545A | c.1634A>C | |
| p.E545G | c.1634A>G | |
| p.E545V | c.1634A>T | |
| Q546 | p.Q546K | c.1636C>A |
| p.Q546E | c.1636C>G | |
| p.Q546P | c.1637A>C | |
| p.Q546R | c.1637A>G | |
| p.Q546L | c.1637A>T | |
| D549 | p.D549N | c.1645G>A |
| PTEN | ||
| R233 | p.R233* | c.697C>T |
| R159S | p.R159S | c.477G>T |
| K267fs*9 | p.K267fs*9 | c.800delA |
| AKT | ||
| E17K | p.E17K | c.49G>A |
RESULTS
Study Population
Fifty-one patients were enrolled from August 2010 to January 2012 (Table 2). Overall, 90% of the patients had bone metastasis and approximately 50% had visceral and/or serosal metastasis. Ninety percent of patients in both groups were previously exposed to at least one line of endocrine therapy for metastatic disease, and 50% and 69% of patients previously received an aromatase inhibitor in the metastatic setting on the continuous and intermittent arms, respectively. Intermittent arm patients had greater exposure to previous chemotherapy regimens in the metastatic setting compared with continuous arm patients (42% v 15%, respectively), and had a shorter median interval between original diagnosis of breast cancer and development of metastatic disease (median of 29 v 76 months, respectively). However, more patients in the intermittent arm had secondary endocrine therapy resistance (74% v 55% of patients, respectively).
Table 2.
Patient Baseline Characteristics and Tumor PIK3CA Status
| Characteristic | Continuous B + L (N = 20) |
Intermittent B + L (N = 31) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age | ||||
| Median | 55 | 56 | ||
| Range | 34-77 | 38-77 | ||
| Time since original diagnosis, months | ||||
| Median | 76 | 29 | ||
| Range | 0-123 | 0-265 | ||
| Metastatic lesions | ||||
| Bone | 19 | 95 | 27 | 87 |
| Visceral | ||||
| Liver | 5 | 25 | 16 | 51 |
| Lung | 3 | 15 | 6 | 19 |
| Pleura/peritoneum | 2 | 10 | 6 | 19 |
| Other | ||||
| Lymph nodes | 1 | 5 | 10 | 32 |
| Skin | 0 | — | 2 | 6 |
| Prior therapies | ||||
| Number of previous therapies | ||||
| Median | 4 | 4 | ||
| Range | 1-11 | 1-13 | ||
| Endocrine therapy in the metastatic setting | 18 | 90 | 28 | 90 |
| Previous aromatase inhibitor therapy | ||||
| Any time | 18 | 90 | 25 | 80 |
| In the metastatic setting | 10 | 50 | 21 | 69 |
| Primary endocrine therapy resistance | 9 | 45 | 8 | 26 |
| Secondary endocrine therapy resistance | 11 | 55 | 23 | 74 |
| Chemotherapy in the metastatic setting | 3 | 15 | 13 | 42 |
| PIK3CA/AKT mutation status in the tumor | ||||
| PIK3CA_pE545K | 2 | 1 | 4 | 13 |
| PIK3CA_pE542K | 1 | 0.5 | 2 | 6 |
| PIK3CA_pH1047R | 1 | 0.5 | 5 | 16 |
| AKT1_pE17K | 1 | 0.5 | — | — |
| Total | 5 | 20 | 11 | 35 |
Abbreviation: B + L, buparlisib + letrozole.
Evaluable tumor samples were obtained in all 51 patients; the majority of these were paraffin-embedded blocks from the original diagnosis (41 from the primary tumor and 10 from a metastatic biopsy). PI3K-pathway mutations were found in five of 20 patients (25%) in the continuous arm, and 11 of 31 patients(35%) in the intermittent arm (Table 2).
Dose De-escalation and MTD
Buparlisib dose was started at 100 mg/d in both treatment arms. DLTs occurred in three of 51 patients: two patients on the continuous arm (transient grade 3 transaminitis that resolved within 3 weeks of buparlisib interruption; drug not reinitiated) and one patient in the intermittent arm (reversible grade 3 depression that resolved within 2 weeks of buparlisib interruption; drug reinitiated at 80 mg). No dose de-escalations were necessary in either treatment arms. The buparlisib MTD in both treatment arms was 100 mg/d. Intrapatient dose reductions occurred in six patients in the continuous arm (four in cycle 2, two beyond cycle 2); and in five patients in the intermittent arm (all in cycle 2). Less than 25% of patients on either arm required buparlisib interruption or dose reduction during the first 8 weeks of treatment.
Safety Findings
Overall, the combination of buparlisib and letrozole was well tolerated (Table 3). Most common adverse effects were GI disorders (80%), transaminitis and hyperglycemia (60% each), and mood disorders (45%). Grade 3 adverse events, regardless of causality, were observed in 14 patients (27%). No grade 4 adverse events were observed. Cumulative grade 3 adverse events between both treatment arms differed slightly (Table 3).
Table 3.
Total Adverse Events by Treatment Arm
| Toxicity Category/Toxicity (CTCAE version 4) | Continuous B + L (N = 20) |
Intermittent B + L (N = 31) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total |
Grade 3 |
Total |
Grade 3 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| GI disorders | ||||||||
| Nausea | 13 | 65.0 | 8 | 25.8 | ||||
| Diarrhea | 10 | 50.0 | 11 | 35.5 | ||||
| Mucositis (oral) | 3 | 15.0 | 2 | 6.5 | ||||
| Vomiting | 3 | 15.0 | 4 | 12.9 | ||||
| Constipation | 4 | 20.0 | 7 | 22.6 | ||||
| General disorders | ||||||||
| Fatigue | 14 | 70.0 | 1 | 5 | 13 | 41.9 | ||
| Investigations | ||||||||
| Transaminase elevation | 15 | 75.0 | 3 | 15 | 14 | 45.2 | 3 | 9 |
| Alkaline phosphatase increased | 12 | 60.0 | 6 | 19.4 | ||||
| Metabolism and nutrition disorders | ||||||||
| Hyperglycemia | 14 | 70.0 | 2 | 10 | 15 | 48.4 | ||
| Anorexia | 4 | 20.0 | 9 | 29.0 | ||||
| Skin and subcutaneous tissue disorders | ||||||||
| Rash maculopapular | 8 | 40.0 | 1 | 5 | 9 | 29.0 | 1 | 3 |
| Pruritus | 7 | 35.0 | 5 | 16.1 | ||||
| Rash acneiform | 7 | 35.0 | 1 | 3.2 | ||||
| Dry skin | 4 | 20.0 | 1 | 3.2 | ||||
| Psychiatric (mood) disorders | ||||||||
| Anxiety | 9 | 45.0 | 1 | 5 | 13 | 41.9 | ||
| Depression | 11 | 55.0 | 1 | 5 | 10 | 32.3 | 1 | 3 |
| Irritability | 4 | 20.0 | ||||||
| Nervous system disorders | ||||||||
| Dizziness | 4 | 20.0 | 6 | 19.4 | ||||
| Ataxia | 3 | 15.0 | ||||||
| Concentration impairment | 2 | 10.0 | ||||||
| Blood and lymphatic system disorders | ||||||||
| Anemia | 11 | 55.0 | 6 | 19.4 | ||||
Abbreviations: B + L, buparlisib + letrozole; CTCAE, Common Terminology Criteria for Adverse Events.
Mood alterations (including anxiety, depression, emotional lability, hallucinations, irritability, and affective disorder) resolved within 2 weeks of buparlisib interruption in severe cases, or controlled with administration of selective serotonin reuptake inhibitors in mild to moderate cases. Of all patients requiring interruption of buparlisib for mood alterations, only one eventually discontinued buparlisib permanently.
Radiographic Assessments
Forty-six of 51 patients (90%) were evaluable for best response (first postbaseline target lesion radiologic assessment) by investigator review (Fig 1). Two of 20 patients (10%) in the continuous arm achieved a complete and a PR, respectively. Each patient had only one previous line of endocrine therapy (tamoxifen and fulvestrant in the metastatic setting, respectively). Both of these patients had wild-type PIK3CA/ER-positive breast cancer. Eleven patients (55%) in the continuous arm had SD; of those, six patients (30%) had SD ≥ 6 months. None of the patients in the intermittent arm had a response, but 14 (45%) of them had SD; of those, 10 (32%) had SD ≥ 6 months. Clinical benefit rate (lack of disease progression ≥ 6 months) was similar for both treatment arms (30%). Of seven patients with SD ≥ 12 months (two in the continuous arm; five in the intermittent arm), three had a PIK3CA hot-spot mutation in their tumor, and all had previous exposure to an aromatase inhibitor in the metastatic setting. Of 16 patients with SD ≥ 6 months (six in the continuous arm; 10 in the intermittent arm), only four were considered to have primary endocrine therapy resistance (Fig 1).
Fig 1.
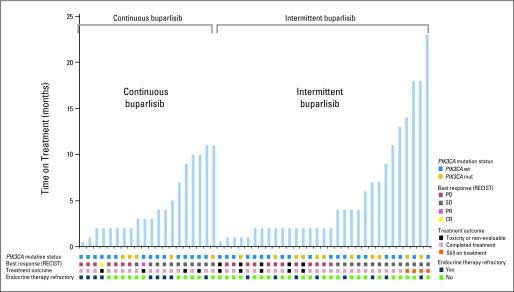
Treatment duration (months) for patients exposed to continuous or intermittent buparlisib plus letrozole, with corresponding radiologic response and tumor PI3K pathway mutation status. Gold shading indicates presence of a PIK3CA mutation, and blue shading indicates no alteration. Best response by RECIST is depicted in gold (complete response [CR]), pink (partial response [PR]), gray (stable disease [SD]), and red (progression of disease [PD]) shading. Black shading indicates discontinuation of treatment because of toxicity or withdrawal; orange shading indicates the four patients who are still on active treatment, and light brown shading corresponds to all patients who discontinued treatment because of disease progression. Dark blue shading indicates patients who had primary endocrine therapy, and light green shading indicates patients who had secondary endocrine therapy resistance.
[18F]FDG-PET/CT Scan Metabolic Assessments
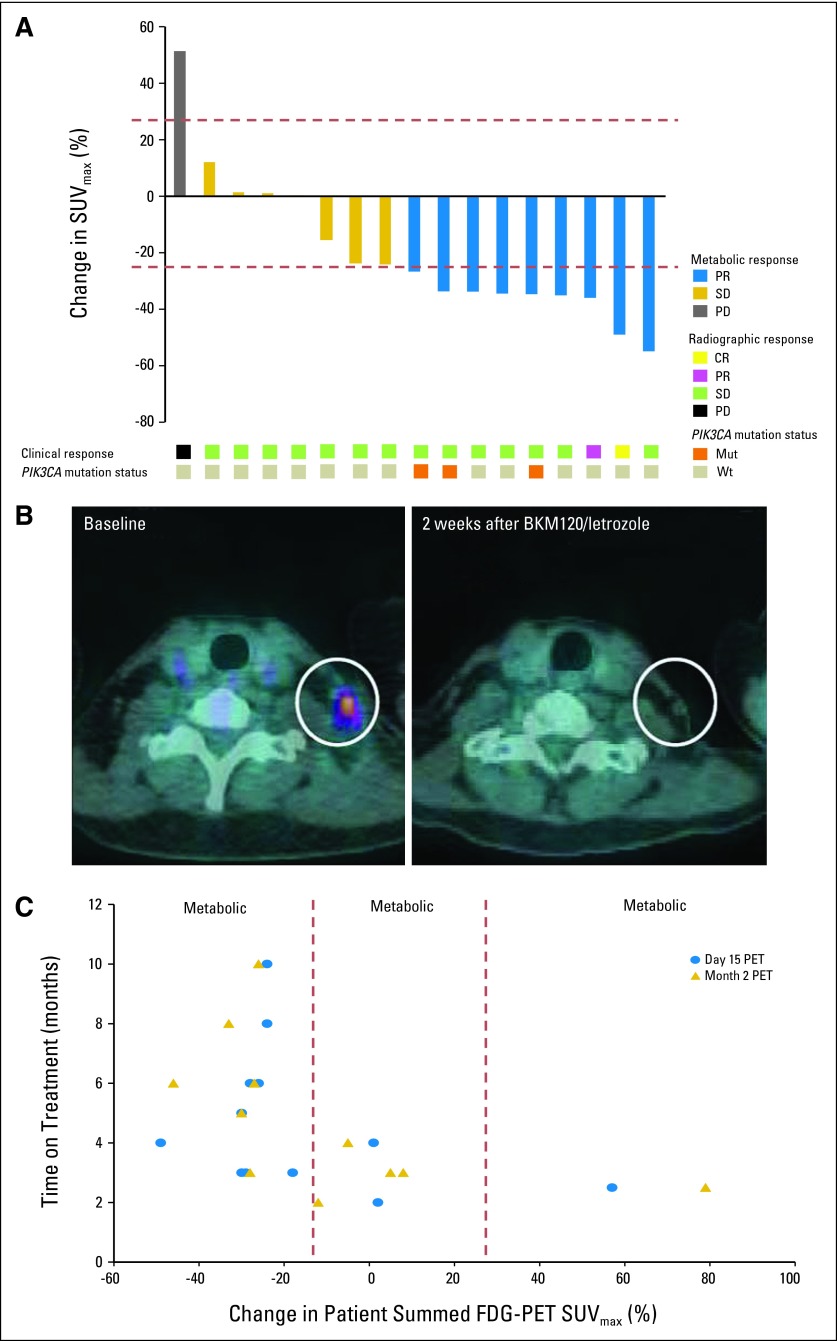
Seventeen of 20 patients (85%) on the continuous arm were evaluable for [18F]FDG-PET/CT assessments (Fig 2). A ≥ 25% decrease in tumor [18F]FDG uptake 15 days after treatment initiation was observed in nine patients by central review (metabolic PR), two of whom demonstrated tumor shrinkage on radiographic assessment by RECIST criteria after 2 months on treatment (Figs 2A and 2B). The day 15 and month 2 metabolic response assessments revealed a strong concordance by Schmidt criteria (Kendall's W = 0.85). Three of the nine patients who had a metabolic PR had a PIK3CA mutation in their tumor. Interestingly, patients who had a metabolic PR in their day 15 and month 2 [18F]FDG-PET/CT scan were also more likely to stay on treatment for a longer duration of time on the basis of lack of radiographic progression (Fig 2C). Conversely, the patient demonstrating metabolic progressive disease at day 15 progressed radiographically at month 2.
Fig 2.
[18F]fluorodeoxyglucose–positron emission tomography/computed tomography ([18F]FDG-PET/CT) metabolic response assessments in patients exposed to continuous buparlisib (BKM120) plus letrozole, with corresponding response by RECIST and tumor PIK3CA status. (A) Seventeen patients in the continuous buparlisib plus letrozole arm were evaluable for [18F]FDG-PET/CT assessments. More than 25% decrease in tumor [18F]FDG uptake 15 days after treatment initiation corresponded to a metabolic partial response (PR; blue shading), which was seen in nine patients. More than 25% increase in tumor [18F]FDG uptake 15 days after treatment initiation corresponded to a metabolic progression of disease (PD; gray shading), which was seen in one patient. All remaining seven patients had metabolic stable disease (SD; gold shading). Two of the patients who exhibited a metabolic partial response 15 days after treatment initiation, one of them exemplified in (B), also had radiographic responses by CT (yellow and pink shading), at their 2-month tumor assessment. All patients with a PIK3CA mutation (orange shading) had metabolic partial responses. (C) Patients who had ≥ 25% reduction in tumor [18F]FDG uptake (metabolic partial response) on day 15 of treatment relative to pretreatment baseline (blue dots), as well as 8 weeks after treatment initiation (gold triangles), had a longer time on treatment than patients with < 25% decrease in tumor [18F]FDG uptake (metabolic SD or metabolic PD), both on day 15 and 8 weeks after treatment initiation. SUVmax, maximum standardized uptake value.
DISCUSSION
The results from this study provide evidence that buparlisib plus letrozole is safe, tolerable, and active in postmenopausal patients with ER-positive/human epidermal growth factor receptor 2–negative metastatic breast cancer refractory to endocrine therapy. The MTD and recommended dose for phase II trials of buparlisib in combination with letrozole was defined as 100 mg/d. At this dose, both administration schedules (continuous or intermittent) were considered tolerable, because > 75% of patients in each group tolerated therapy for ≥ 8 weeks without development of grade ≥ 2 GI disturbances, hyperglycemia, rash, or mood alterations.
Most common adverse effects (hyperglycemia, nausea, diarrhea, fatigue, and mood disorders) in the continuous dosing of buparlisib with letrozole were of similar frequency to those in the phase I study of buparlisib single agent.15 They were also consistent with what has been seen with other PI3K pathway inhibitors.18–20 However, the rates of transaminitis in the intermittent schedule dosing were lower, probably because of reduced buparlisib accumulation. All cases of transaminitis resolved within 2 weeks with buparlisib interruption and, when appropriate, with dose reduction.
The intermittent schedule had lower rates of all common adverse effects, including hyperglycemia. This was to be expected because hyperglycemia is a class effect, and more commonly seen on more sustained inhibition of p110α.15 Importantly, none of the patients required administration of insulin to manage their hyperglycemia; all of them were managed with metformin. We postulate that by managing buparlisib-induced hyperglycemia without resorting to insulin, we could circumvent the stimulation of insulin receptors (and thus PI3K) in breast cancers.21
Buparlisib can cross the blood-brain barrier (data on file, Novartis, New York, NY), potentially inhibiting PI3K in the CNS22,23; this effect could result in anxiety, depression, and irritability. The incidence of mood alterations in patients exposed to the combination of letrozole and daily buparlisib was similar to what has been reported in the phase I single agent buparlisib trial (40% to 50%). Mood alterations were overall mild to moderate, and responsive to dose-reductions/interruptions (suggesting dose-dependency), as well as treatment with selective serotonin reuptake inhibitors and anxiolytics.
Over 50% of patients undergoing [18F]FDG-PET/CT scan exhibited a reduction in FDG tumor uptake at 2 and 8 weeks post-treatment initiation. Although this likely reflects pharmacodynamic effects of the PI3K inhibitor,13,15 there was a correlation between the magnitude of the decrease in FDG-uptake relative to baseline and the duration of treatment, suggesting the decrease in tumor metabolism may predict response to therapy. This speculation is also supported by the observation in one patient where increase in FDG uptake preceded rapid disease progression.
The combination of letrozole and buparlisib revealed clinical activity regardless of administration schedule of the PI3K inhibitor. Approximately 30% of patients remained on treatment ≥ 6 months, and 7 of 51 patients remained on treatment ≥ 12 months. Interestingly, the clinical benefit rate seen in this study is not inconsistent with the clinical benefit rate in patients with ER-positive metastatic breast cancer treated with exemestane and the mTORC1 inhibitor everolimus in the BOLERO-2 phase III study.24 Additionally, the benefit of buparlisib addition to letrozole was mostly seen in patients with secondary endocrine therapy resistance, similar to the pattern seen in patients with ER-positive metastatic breast cancer treated with tamoxifen and everolimus in the phase II TAMRAD study.25 This clinical activity is consistent with reports revealing that hyperactivation of the PI3K/AKT pathway confers adaptation to estrogen deprivation in experimental models of hormone-dependent breast cancer. In these studies, PI3K pathway inhibitors trump this adaptation of ER-positive cells to estrogen deprivation and/or synergize with antiestrogens in inducing an antitumor effect.9–11,26 It remains to be determined if p110α-specific inhibitors would have greater activity against PIK3CA-mutant tumors. To start to address this question, several phase Ib clinical trials of p110α-specific inhibitors in combination with endocrine therapy in patients with/without PIK3CA mutations are close to completion.
The majority (90%) of PIK3CA mutation analysis were performed in paraffin-embedded blocks from primary tumors. Several studies have addressed discordance rates in PIK3CA mutation between primary tumor and recurrence/metastasis, but these varied substantially from study to study.27,28 A recent, larger analysis performed exclusively in ER-positive breast cancer examined PIK3CA mutations in 88 paired-matched samples at both diagnosis and recurrence.29 Frequent changes in PIK3CA mutation status were seen in patients who developed a new primary breast cancer. However, few patients (< 10%) changed their PIK3CA mutation status in a local or a metastatic recurrence, or postprogression on endocrine treatment, suggesting that PIK3CA mutation status at diagnosis can be used to determine eligibility or stratification within a clinical trial with a PI3K pathway inhibitor.
Buparlisib and letrozole clinical activity did not correlate with PIK3CA hot-spot mutations. Approximately 50% of the patients who had no disease progression ≥ 12 months had a PIK3CA-mutated cancer. The fact that wild-type PIK3CA breast cancers also appeared to benefit from buparlisib plus letrozole suggests that these cancers harbor other alterations in the PI3K pathway, resulting in PI3K dependence. Recently published deep sequencing data30 suggest the coexistence of mutations and/or amplifications in several known driver genes, in addition to PIK3CA mutations. One trial patient was found to have an AKT1 activating mutation. However, she did not have a metabolic response by [18F]FDG-PET/CT scan and developed disease progression after 3 months of therapy. This example supports the notion that an activating mutation in AKT1 would cause it to signal independent of phosphatidylinositol (3,4,5) - triphosphate. As such, tumors with this alteration should not be sensitive to a PI3K inhibitor.
To our knowledge, this is the first phase Ib trial of an antiestrogen with a PI3K inhibitor in breast cancer. The results of this study demonstrate the combination of the pan-class I PI3K inhibitor buparlisib with letrozole as safe, tolerable, and active in patients with endocrine therapy-resistant ER-positive advanced breast cancer, particularly those with secondary endocrine therapy resistance. Phase II and III trials of endocrine therapy with/without buparlisib are ongoing.
Acknowledgment
We thank the patients for their participation, and their families for support throughout the study. We also thank the whole American Association for Cancer Research/Stand Up to Cancer Dream Team–PI3K Pathway in Women's Cancers for academic contributions.
Glossary Terms
- CYP3A4:
gene encoding cytochrome P450, subfamily IIIA (niphedipine oxidase), polypeptide 4. Notably present in the liver, the mixed-function oxidase is the most important enzyme involved in the metabolism of xenobiotics and oxidizes a wide range of substrates, including taxanes.
- AKT:
a transforming serine-threonine kinase involved in cell survival.
- estrogen receptor (ER):
ligand-activated nuclear proteins, belonging to the class of nuclear receptors, present in many breast cancer cells that are important in the progression of hormone-dependent cancers. After binding, the receptor-ligand complex activates gene transcription. There are two types of estrogen receptors (ERα and ERβ). ERα is one of the most important proteins controlling breast cancer function. ERβ is present in much lower levels in breast cancer, and its function is uncertain. Estrogen receptor status guides therapeutic decisions in breast cancer.
- mammalian target of rapamycin (mTOR):
member of a protein complex (along with raptor and GβL) that is used by cells to sense nutrients in the environment. mTOR is a serine/threonine kinase that is activated by AKT and regulates protein synthesis on the basis of nutrient availability. It was discovered when rapamycin, a drug used in transplantation, was shown to block cell growth presumably by blocking the action of mTOR.
- mTORC1:
complex composed of mammalian target of rapamycin (mTOR), regulatory associated protein of mTOR (raptor), and mLST8/GL. This complex has the classic features of mTOR by functioning as a sensor for nutrients and energy and controlling protein synthesis. The complex is downstream from AKT and phosphorylates S6K1 upon activation.
- pharmacokinetics:
a branch of pharmacology that studies the relationship between drug exposure level, time course of exposure, and the overall response of an organism. Although pharmacokinetics is largely applied to drugs, it is also applicable to other compounds such as nutrients, toxins, hormones, etc. Pharmacokinetics is subdivided into absorption and disposition (distribution, metabolism, and excretion) and is generally referred to as ADME (absorption, distribution, metabolism, excretion). With respect to drugs administered, all processes occur in tandem once a drug dose is administered. In clinical trials, phase I studies will typically study pharmacokinetics and safety of the drug.
- PI3K/AKT pathway:
signal transduction pathways involving the signaling molecules phosphatidylinositol-3 kinase (PI3K) and AKT, where PI3K generates phosphorylated inositides at the cell membrane, which are required for the recruitment and activation of AKT, a transforming serine-threonine kinase involved in cell survival.
- PIK3CA:
the catalytic subunit of phosphatidylinositol 3-kinase involved in the generation of PIP3 which, in turn, leads to the activation of AKT and other oncogenic kinases. Mutations in the PIK3CA gene have been found in several cancers, including ovarian, breast, colon, and lung carcinomas.
Appendix
Patients and Methods
Mutation analysis.
Snapshot analysis is based on multiplex polymerase chain reaction, primer extension with fluorescently tagged dideoxy-nucleotides and capillary electrophoresis; it is a fast, high-throughput, multiplex profiling method on the basis of the Applied Biosystems Snapshot platform.17 Mutations can be detected when mutant DNA comprises as low as 5% of the total tumor DNA.
[18F]FDG-PET/CT Scan Assessments
To assess the functional tumor burden, we first defined measurable functional lesions on [18F]fluorodeoxyglucose-positron emission tomography/computed tomography ([18F]FDG-PET/CT) scan as the most metabolically active lesions; ie, those with the highest FDG uptake as defined by the highest standardized uptake value (SUV). We measured up to five of these lesions per patient, and assessed the functional tumor burden by summing these lesions (as it would be done with Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1,31 where the sum of diameters of the target lesions defines the anatomic tumor burden). The potential advantage of the sum of maximum SUV (SUVmax) over the SUVmax of a single lesion or the maximum over several lesions is that the sum of SUVmax is more stable than the maximum SUVmax. This sum incorporates more comprehensively the various metabolically active sites of disease burden, therefore better reflecting the overall anatomic tumor burden.32
Footnotes
Supported by Stand Up to Cancer Dream Team Translational Research Grant, Program of the Entertainment Industry Foundation (Grant No. SU2C-AACR-DT0209), Breast Cancer Specialized Program of Research Excellence Grant No. P50 CA098131, Vanderbilt-Ingram Cancer Center Support Grant No. P30 CA68485, Breast Cancer Research Foundation grant (C.L.A.), Susan G. Komen for the Cure Foundation SAC Grant No. SAC100013, Grant No. K23 CA127469-01A2 (I.A.M.), and by Grant No. R01 GM041890 (L.C.C.). This study was sponsored by Novartis Pharmaceuticals.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Presented in part at the 48th Annual American Society of Clinical Oncology Meeting, Chicago, IL, June 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01248494.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Ingrid A. Mayer, Novartis (C); Lewis C. Cantley, Novartis (C); Eric Winer, Novartis (U) Stock Ownership: None Honoraria: None Research Funding: Ingrid A. Mayer, Novartis; Andres Forero, Novartis Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Ingrid A. Mayer, Yisheng Li, Lewis C. Cantley, Eric Winer, Carlos L. Arteaga
Financial support: Ingrid A. Mayer, Lewis C. Cantley, Carlos L. Arteaga
Administrative support: Ingrid A. Mayer, Carlos L. Arteaga
Provision of study materials or patients: Ingrid A. Mayer, Vandana G. Abramson, Steven J. Isakoff, Andres Forero, Eric Winer, Carlos L. Arteaga
Collection and assembly of data: Ingrid A. Mayer, Justin M. Balko, María Gabriela Kuba, Melinda E. Sanders, Jeffrey T. Yap, Annick D. Van den Abbeele, Yisheng Li, Eric Winer, Carlos L. Arteaga
Data analysis and interpretation: Ingrid A. Mayer, Vandana G. Abramson, Steven J. Isakoff, Andres Forero, Justin M. Balko, Melinda E. Sanders, Jeffrey T. Yap, Annick D. Van den Abbeele, Yisheng Li, Lewis C. Cantley, Eric Winer, Carlos L. Arteaga
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorn PJ, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarty A, Rexer BN, Wang SE, et al. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29:5193–5203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Tenorio G, Alkhori L, Olsson B, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 6.Loi S, Haibe-Kains B, Majjaj S, et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci USA. 2010;107:10208–10213. doi: 10.1073/pnas.0907011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–4461. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowder RJ, Phommaly C, Tao Y, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69:3955–3962. doi: 10.1158/0008-5472.CAN-08-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller TW, Balko JM, Fox EM, et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discovery. 2011;1:338–351. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(suppl 1):12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 13.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 14.Maira SM, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 15.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 16.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 17.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: A phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 19.Edelman G, Bedell C, Shapiro G. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28:234s. (abstr 3004) [Google Scholar]
- 20.Brana I, Lorusso PM, Baselga J. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;28:240s. (abstr 3030) [Google Scholar]
- 21.Fox EM, Miller TW, Balko JM, et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011;71:6773–6784. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackermann TF, Hortnagl H, Wolfer DP, et al. Phosphatidylinositide dependent kinase deficiency increases anxiety and decreases GABA and serotonin abundance in the amygdala. Cell Physiol Biochem. 2008;22:735–744. doi: 10.1159/000185557. [DOI] [PubMed] [Google Scholar]
- 23.Kalkman HO. The role of the phosphatidylinositide 3-kinase-protein kinase B pathway in schizophrenia. Pharmacol Ther. 2006;110:117–134. doi: 10.1016/j.pharmthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO study. J Clin Oncol. 2012;30:2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 26.Fox EM, Kuba MG, Miller TW, et al. Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation. Breast Cancer Res. 2013;15:R55. doi: 10.1186/bcr3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akcakanat A, Sahin A, Shaye AN, et al. Comparison of Akt/mTOR signaling in primary breast tumors and matched distant metastases. Cancer. 2008;112:2352–2358. doi: 10.1002/cncr.23456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10:1093–1101. doi: 10.1158/1535-7163.MCT-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon JM, Renshaw L, Keys J, et al. PI3-kinase mutations in recurrences in patients with estrogen receptor positive breast cancer. San Antonio Breast Cancer Symposium; 2013; San Antonio, TX. (poster discussion 1:01) [Google Scholar]
- 30.Ellis MJ, Ding L, Shen D, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353–360. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Jadvar H, Desai B, Ji L, et al. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. doi: 10.2967/jnumed.112.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]