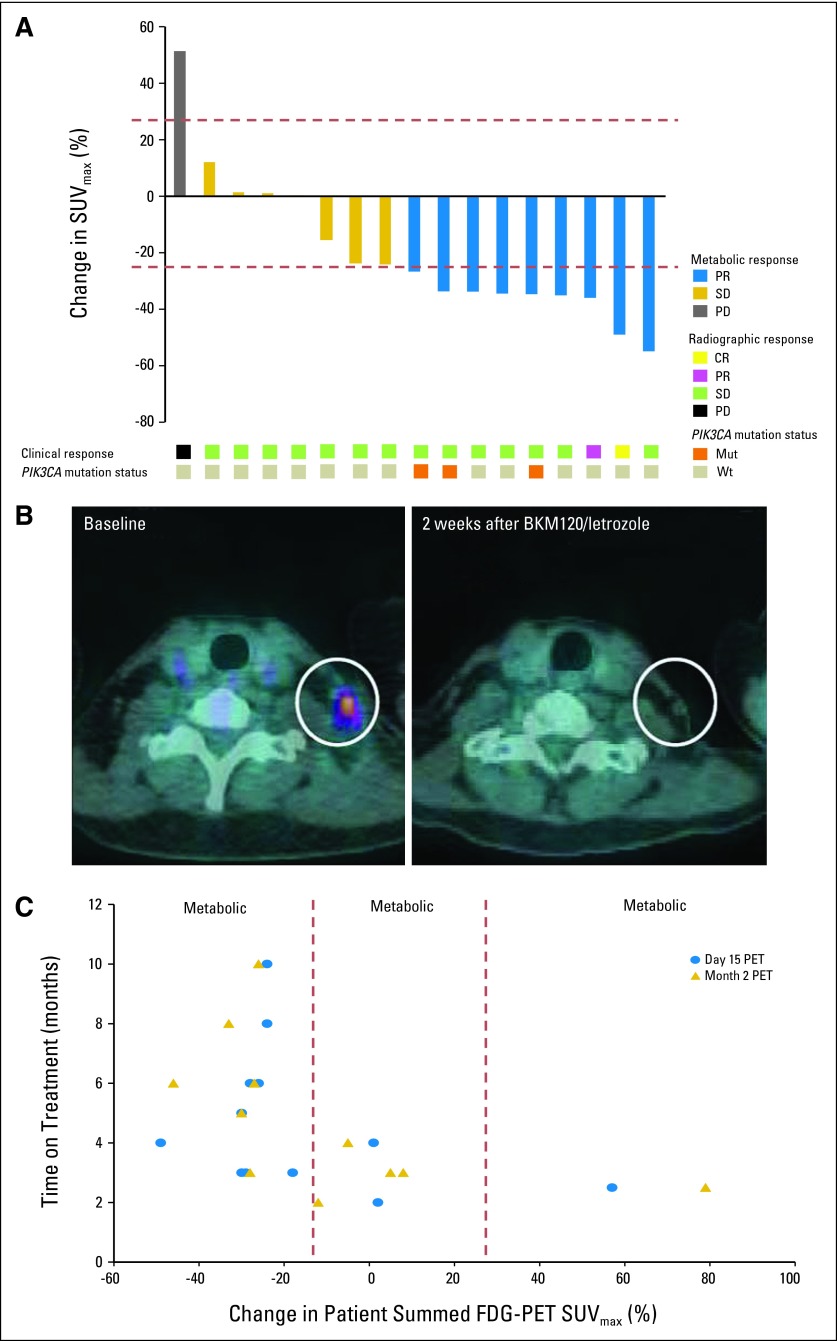
Fig 2.
[18F]fluorodeoxyglucose–positron emission tomography/computed tomography ([18F]FDG-PET/CT) metabolic response assessments in patients exposed to continuous buparlisib (BKM120) plus letrozole, with corresponding response by RECIST and tumor PIK3CA status. (A) Seventeen patients in the continuous buparlisib plus letrozole arm were evaluable for [18F]FDG-PET/CT assessments. More than 25% decrease in tumor [18F]FDG uptake 15 days after treatment initiation corresponded to a metabolic partial response (PR; blue shading), which was seen in nine patients. More than 25% increase in tumor [18F]FDG uptake 15 days after treatment initiation corresponded to a metabolic progression of disease (PD; gray shading), which was seen in one patient. All remaining seven patients had metabolic stable disease (SD; gold shading). Two of the patients who exhibited a metabolic partial response 15 days after treatment initiation, one of them exemplified in (B), also had radiographic responses by CT (yellow and pink shading), at their 2-month tumor assessment. All patients with a PIK3CA mutation (orange shading) had metabolic partial responses. (C) Patients who had ≥ 25% reduction in tumor [18F]FDG uptake (metabolic partial response) on day 15 of treatment relative to pretreatment baseline (blue dots), as well as 8 weeks after treatment initiation (gold triangles), had a longer time on treatment than patients with < 25% decrease in tumor [18F]FDG uptake (metabolic SD or metabolic PD), both on day 15 and 8 weeks after treatment initiation. SUVmax, maximum standardized uptake value.