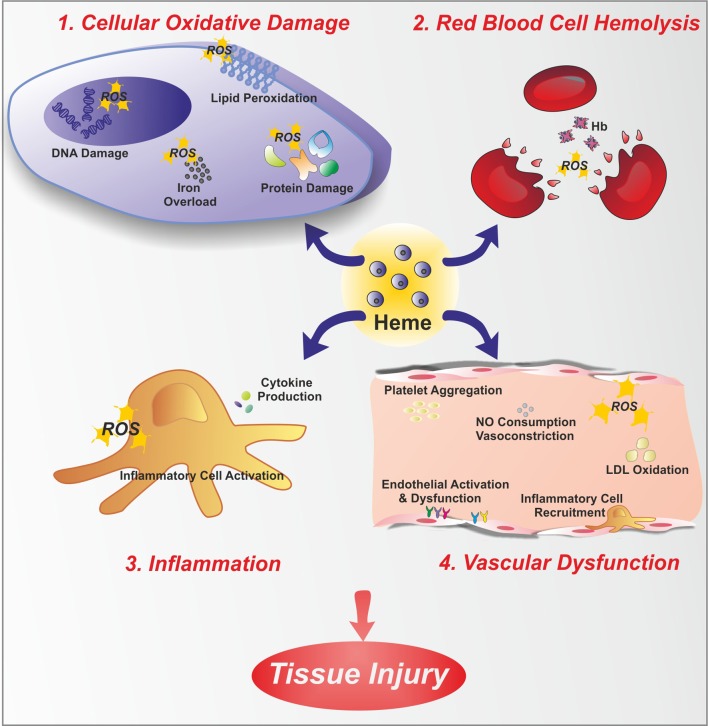
Figure 1.
Free heme toxicity. Free heme has potentially toxic properties due to the catalytic active iron atom it coordinates. Here, toxic effects of heme are depicted. Heme causes cellular oxidative damage (1) by promoting ROS formation, lipid peroxidation, DNA and protein damage. Additionally, heme is a source of iron. Therefore, heme overload leads to intracellular accumulation of iron, with further ROS generation. Heme is a hemolytic agent (2), since it intercalates red blood cell membrane, thus favoring cell rupture and further amplifying the hemolytic process. Heme promotes inflammation (3), by stimulating inflammatory cell activation and cytokine production. Finally, heme causes endothelial dysfunction (4) by several mechanisms: increasing adhesion molecule expression and endothelial activation, promoting inflammatory cell recruitment and platelet aggregation, causing nitric oxide (NO) oxidative consumption and vasoconstriction, oxidizing low-density lipoprotein (LDL).