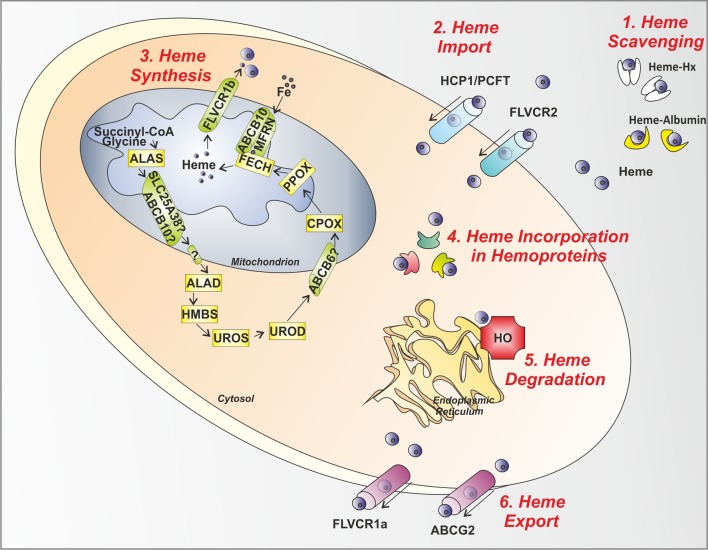
Figure 2.
Control steps in heme metabolism. The main mechanisms involved in the control of heme levels outside, inside and across the cell are illustrated. (1) Heme scavenging: Circulating free heme toxicity is avoided thanks to the action of the scavenging proteins Hx and Albumin. (2) Heme Import: Heme might be imported inside the cell via the putative heme importers HCP1/PCFT and FLVCR2. (3) Heme Synthesis: in the mitochondrion and cytosol, the heme biosynthetic enzymes, starting from succinyl-CoA and glycine, give rise to heme. After synthesis, heme is exported out of the mitochondrion to the cytosol by the mitochondrial heme exporter FLVCR1b. (4) Heme Incorporation in Hemoproteins: once released in the cytosol, heme is inserted in apo-hemoproteins to allow the formation of functional hemoproteins. (5) Heme Degradation: in the endoplasmic reticulum, the heme degrading enzyme HO is responsible for heme degradation into iron (Fe), carbon monoxide and biliverdin. (6) Heme Export: the heme exporters FLVCR1a and ABCG2 regulate heme export out of the cell across the plasma membrane. ALAS, aminolevulinic acid synthase; SLC25A38, solute carrier family 25 member 38; ABCB10, ATP-binding cassette sub-family B member 10; ALAD, amino levulinic acid dehydratase; HMBS, hydroxymethylbilane synthase; UROS, uroporphyrinogen III synthase; UROD, uroporphyrinogen decarboxylase; ABCB6, ATP-binding cassette sub-family B member 6; CPOX, coproporphyrinogen oxidase; PPOX, protoporphyrinogen oxidase; FECH, ferrochelatase; MFRN, mitoferrin; FLVCR, feline leukemia virus subgroup C receptor; HCP1/PCFT heme carrier protein 1/proton-coupled folate transporter; ABCG2, ATP-binding cassette sub-family G member 2; HO, heme oxygenase; Hx, Hemopexin.