Figure 4.
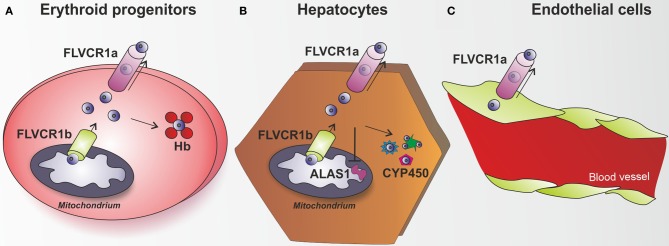
The heme exporter FLVCR1a acts as a new heme detoxifying system. (A) Erythroid progenitors are able to synthesize and handle high amount of heme, in view of their hemoglobin (Hb)-mediated oxygen transport activity. FLVCR1b acts as a mitochondrial heme exporter to allow newly formed heme release from the mitochondrion to the cytosol, where it is incorporated into hemoproteins. FLVCR1a has been described as a system involved in the control of heme levels inside erythroid progenitors. By mediating heme export out of these cells, FLVCR1a regulates intracellular heme amount, thus limiting free heme toxicity and oxidative damage. (B) Hepatocytes have the highest rate of heme synthesis after the erythroid progenitors. Hepatic heme is mostly used for synthesis of P450 enzymes, which metabolize endogenous compounds and xenobiotics. FLVCR1a mediates heme export out of hepatocytes, thus maintaining hepatic heme homeostasis and controlling cell oxidative status. FLVCR1a export function allows the maintenance of a proper cytosolic heme pool that matches cell need for new hemoprotein generation (e.g., cytochrome P450). Block of heme export causes heme pool expansion leading to the inhibition of heme synthesis and the reduction of cytochrome activity. (C) A similar role for FLVCR1a was proposed to occur in endothelial cells. Flvcr1a−/− embryos show reduced vascular arborization, potentially due to altered endothelial integrity, suggesting that the lack of FLVCR1a leads to intracellular heme overload and oxidative stress. Endothelial cells are highly sensitive to heme overload and, in this context, FLVCR1a function could be of crucial importance to export heme excess, thus maintaining heme homeostasis and controlling heme-induced oxidative stress.
