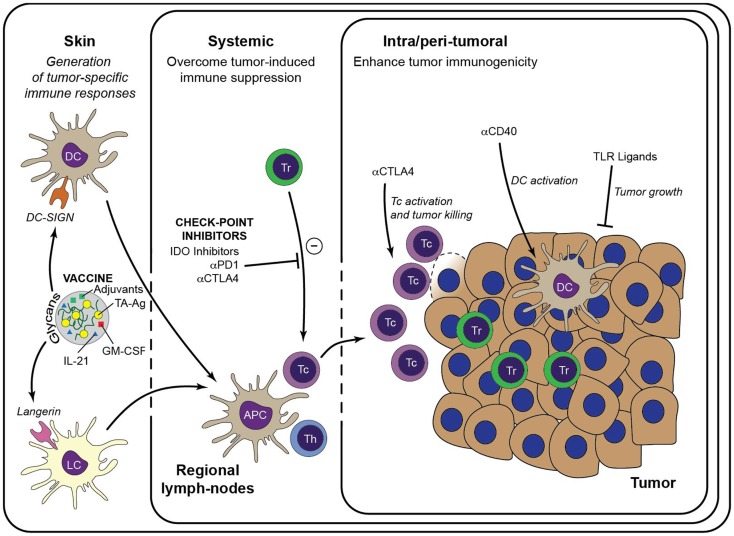
Figure 2.
Immunotherapeutic strategies to enhance anti-tumor immunity. Generation of a large pool of effector TA-specific T cells is induced by the intradermal injection of anti-tumor vaccines. Targeting of the vaccine to a particular skin DC subset is facilitated by modification with specific glycans that bind either to DC-SIGN or Langerin. Subsequent vaccine internalization induces presentation of TA-Ag and maturation of the DCs. Matured DCs migrate to draining lymph nodes to prime TA-specific CD8+ T cells and CD4+ T helper cells, leading to a large pool of cytotoxic effector T cells that are capable to infiltrate the tumor lesion and lyse tumor cells. Priming of TA-specific T cells may be enhanced by inclusion of immunostimulators such as GM-CSF and IL-21 in the DC-targeting vaccine. Systemic or intra-tumoral administered check-point inhibitors, such as anti-PD-1 and anti-CTLA-4, release the break on the anti-tumor immune response by limiting the activity of suppressive Treg and alleviate the priming and/or function of TA-specific CTLs. Similarly, anti-tumor immunity may be enhanced by manipulation of the local micromilieu via administration of DC activating antibodies (e.g., anti-CD40) or of TLR ligands that act directly on the tumor cells. It is anticipated that these strategies may enhance the efficacy of DC-targeted vaccination. Tc, cytotoxic CD8+ T cell; Th, T helper cell.