Figure 5.
FOXO3 Promotes Activation of Notch Signaling, an Essential Pathway for the Maintenance of SC Quiescence
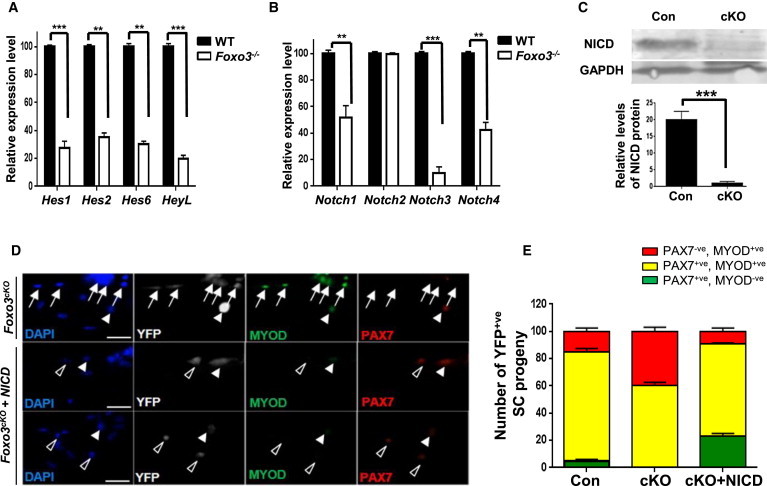
(A and B) FACS-purified QSCs were isolated from Foxo3−/− and wild-type muscles and analyzed for levels of expression of Notch target genes and Notch receptors by qRT-PCR. RNA expression levels in Foxo3−/− SCs are normalized to those in wild-type SCs. Samples represent triplicate experiments of pooled RNA from two mice for each experiment (∗∗∗p < 0.005; ∗∗p < 0.01).
(C) A western blot analysis with an anti-NICD antibody was performed with lysates from FACS-sorted SCs isolated from Foxo3cKO and control mice. Control represents wild-type SCs isolated from littermate offspring that were not treated with tamoxifen. The graph below shows that NICD levels are reduced in Foxo3cKO SCs. GAPDH serves as a loading control. Con and cKO samples represent triplicate experiments of pooled lysates from SCs sorted from two mice for each experiment (∗∗∗p < 0.005).
(D) Single myofibers isolated from EDL muscles from tamoxifen-treated Foxo3cKO mice were infected with retroviral vectors expressing NICD or control virus for 72 hr. Myofibers from EDL muscles from control mice were also isolated. YFP+ve SCs were coimmunostained with PAX7 and MYOD to determine self-renewing (PAX7+ve, MYOD−ve; open arrowheads), proliferating (PAX7+ve, MYOD+ve; closed arrowheads), and differentiating (PAX7−ve, MYOD+ve; arrows) populations. Nuclei were counterstained with DAPI (scale bar represents 100 μm).
(E) The distribution of the three populations of SCs illustrated in (D) was quantified after 72 hr in culture. NICD expression in cKO SCs results in an increase in self-renewing SCs. Data from multiple myofibers were pooled to give a population mean (±SEM) for cells in each category (n = 80 fibers per sample).
