Figure 1.
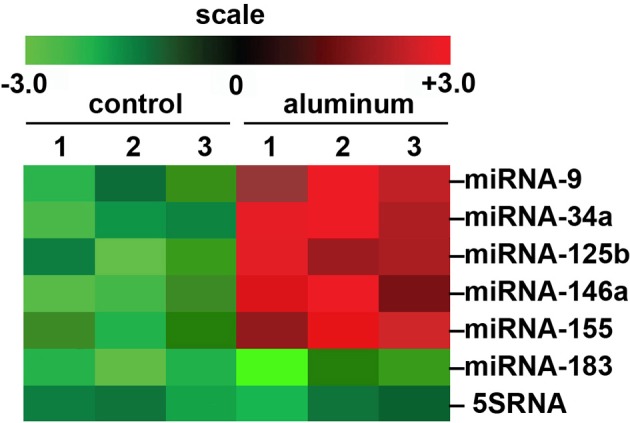
Array-based cluster analysis of miRNA abundance in aluminum-fed Tg2576 mice vs. controls. In these experiments the brain (cortex) of 3 month-old Tg2576 mice fed aluminum-enriched diets were analyzed for miRNA speciation compared to age-matched controls (receiving standard diets); methodologies for aluminum-treatment of transgenic animals have been previously described in detail (Praticò et al., 2002; Zhang et al., 2012). Aluminum treatment of other transgenic murine AD models such as the amyloid over-expressing APP/PS1 show similar intensification of AD-type changes (Zhang et al., 2012). The up-regulation of the pro-inflammatory quintet of miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a, and miRNA-155, depicted here, were amongst the most significantly increased miRNAs found to be 2- to 5-fold above normal diet, age-matched controls (compared to an unchanging internal control miRNA-183 and 5SRNA in the same samples). The results strongly suggest a potential contribution of aluminum to the AD processes associated with miRNA-mediated down-regulation of gene expression in the sporadic AD brain as is widely observed (Colangelo et al., 2002; Lukiw and Pogue, 2007; Ginsberg et al., 2012; Pogue et al., 2012; Alexandrov et al., 2013). Importantly, other common environmental neurotoxic divalent metals such as calcium, cadmium, copper, iron (2+), mercury, nickel, and lead, and neurotoxic trivalent metals such as boron, chromium, gadolinium, indium, iron (3+), and yttrium do not exhibit this potentially pathogenic effect (Walker et al., 1989; Alexandrov et al., 2005; Lukiw, 2010; unpublished observations); N = 3 control and N = 3 aluminum-treated mice; methodologies and data analysis have been extensively described elsewhere (Lukiw and Pogue, 2007; Alexandrov et al., 2013).
