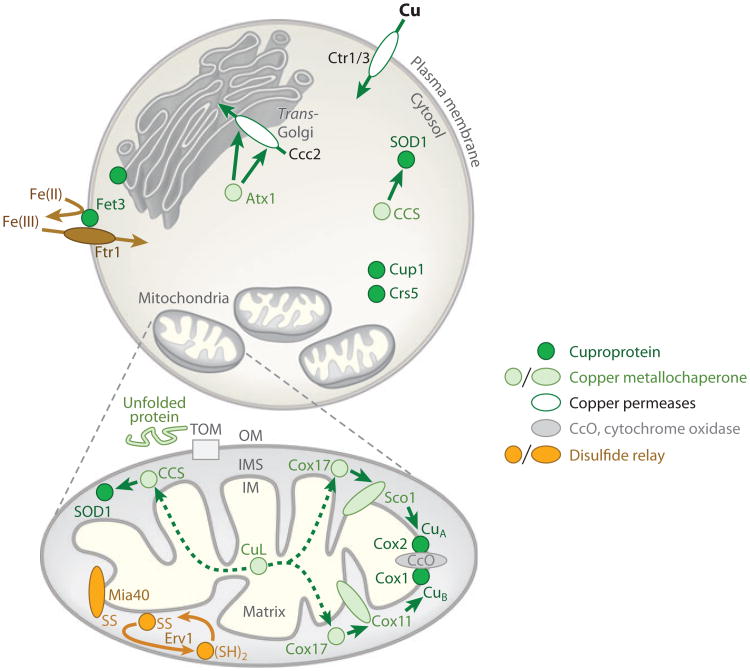
Figure 2.
Copper is passed (green arrows) to cuproproteins (dark green circles), including metallothioneins Crs5 and Cup1, in for example Saccharomyces cerevisiae, by copper metallochaperones (pale green circles, or ovals if membrane associated). Copper permeases (white ovals) import copper into the cell or deliver Cu(I) to compartments, such as the trans-Golgi network where secreted cuproproteins (such as Fet3) acquire copper. Fet3 oxidizes ferrous ions (curved brown arrow) to provide substrate for high-affinity iron import by Ftr1. P1-type ATPases (such as Ccc2) have one or more soluble N-terminal domains, which engage in regulatory interactions with copper metallochaperones (such as Atx1), but additional Cu(I) donation (hence two arrows) may supply the trans-membrane regions for Cu(I) transport. Metallation of the cytochrome c oxidase complex (CcO) in mitochondria (OM, outer membrane of mitochondria) involves a pathway via Cox11 for the CuB site of the Cox1 subunit, embedded in the inner membrane (IM), and via Sco1 for the CuA site of Cox2, protruding into the intermembrane space (IMS). Cox17 supplies both pathways. Nuclear encoded mitochondrial proteins, such as Cox17 and Ccs1, are imported across the OM unfolded (pale green line) via the TOM translocase then captured in the IMS, following introduction of disulfide bonds (SS) through the actions of Mia40. A sulfhydryl oxidase Erv1 generates a reactive disulfide on Mia40. A small copper ligand (CuL) supplies Cu(I) (dashed arrows) to the IMS copper metallochaperones.