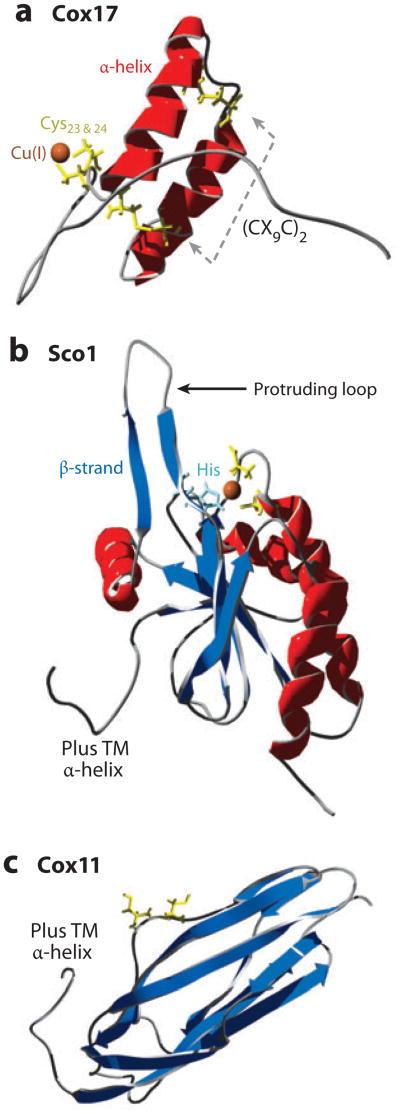
Figure 5.
Copper metallochaperones for CcO. (a) Disulfide bonds between two pairs of cysteine residues (yellow) on antiparallel α-helices (red) create a helical hairpin of Cox17 [Protein Data Bank (PDB) code 2RNB]. Cu(I) is coordinated to a pair of cysteine residues. (b) Sco1 is tethered to the inner membrane (IM) (hydrophobic α-helix not shown) with a thioredoxin fold (β-strand, dark blue; histidine, pale blue) in the intermembrane space (PDB 2GQM). (c) Cox11 is tethered to the IM (hydrophobic α-helix not shown) with an immunoglobulin-like β-barrel in the IMS (PDB 1SPO).