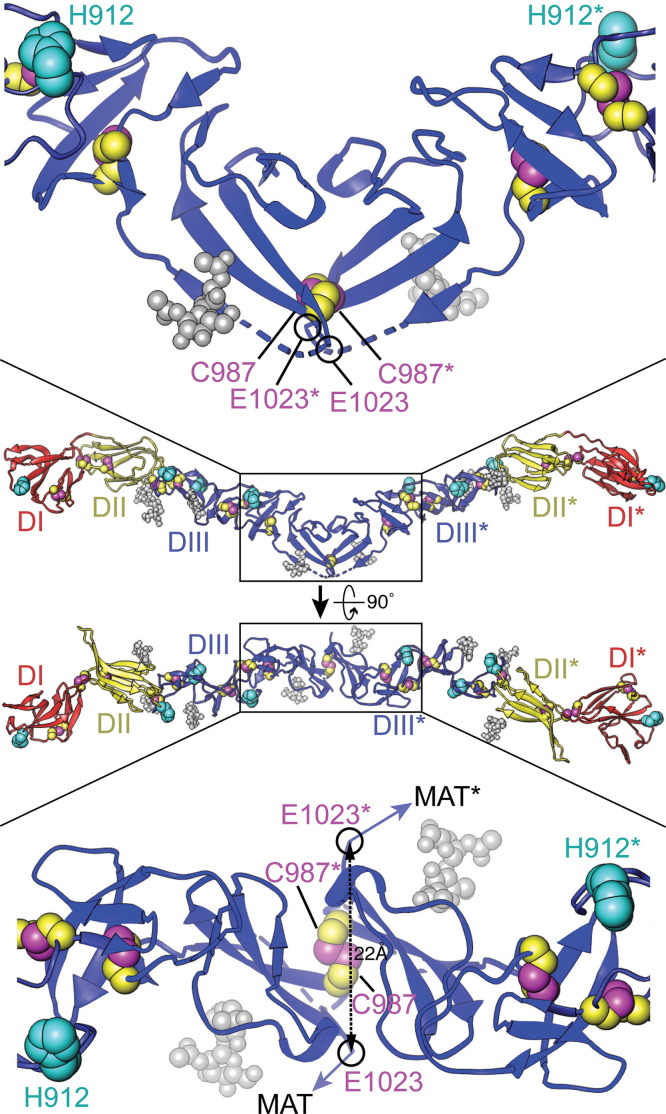
Fig. 1.
Two orthogonal views of the dimeric BVDV E2 ectodomains with close-up views near the dyad. Numbering refers to the polyprotein of BVDV-1 strain NADL. N-linked glycans are shown in gray space-filling representation, His residues (H762, H912, H846, and H871) in cyan, and Cys side chains in magenta (Sγ atoms) and yellow (carbon atoms). The locations of proposed intermolecular disulfide bonds between C987 of E2 and C668 of E1 are indicated by magenta arrows. E2 is colored by domain: I, red; II, yellow; III blue. The most C-terminal residue in the E2 crystal structure, E1023 (circled in black), connects to the membrane anchor and C-terminal tail (MAT). Seven additional C-terminal residues in the crystallized E2 construct were disordered in the crystal, including two conserved His residues (H1027 and H1028) between the membrane anchor and the ectodomain. The distance between the Cα atoms of E1023 from the two subunits is 22 Å. The Cα–Cα distance for C987 from the two subunits is 6.5 Å.