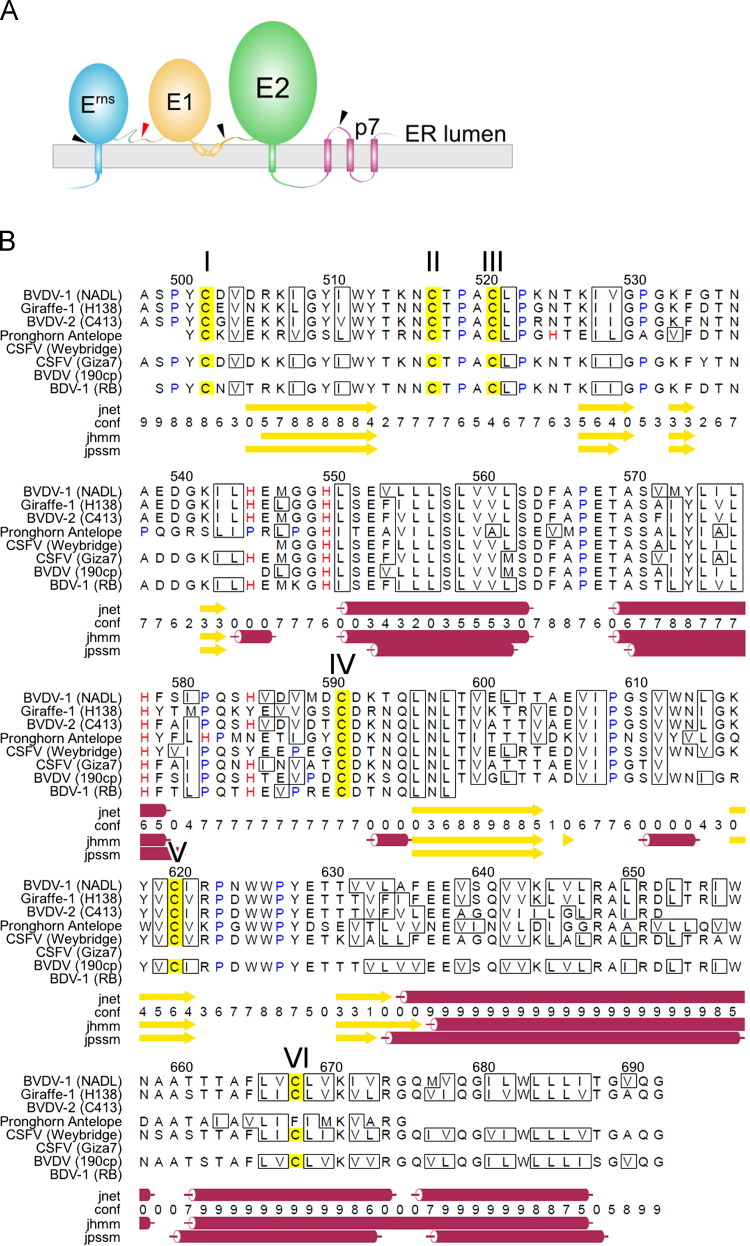
Fig. 3.
Topology and secondary structure predictions of BVDV E1. (A) Topology of the pestivirus E1 and E2 proteins. Wedges indicate the sites at which proteases cleave during polyprotein maturation. (B) Secondary structure prediction of BVDV E1 based on a pestivirus multiple sequence alignment. Secondary structure elements were predicted with Jpred 3. Sequence numbering refers to the BVDV-1 NADL polyprotein. The sequence contains six cysteines, labeled I–VI and referred to in the text as such. Conserved His residues flank the first two predicted α-helices. The following pestivirus E2 UniProt sequences were selected for the sequence alignment based on search results using the BVDV-1 NADL sequence as the query in search against the UniRef90 database: Q9PYB2, pestivirus giraffe-1 strain H138; UPI0000167D3B, BVDV-2 strain C413; Q533M7, pronghorn antelope pestivirus; Q68954, classical swine fever virus strain Weybridge; O90243, classical swine fever virus strain Giza7; Q76B25, bovine viral diarrhea virus strain 190cp; P89049, pestivirus type 3 (border disease virus 1) strain RB.