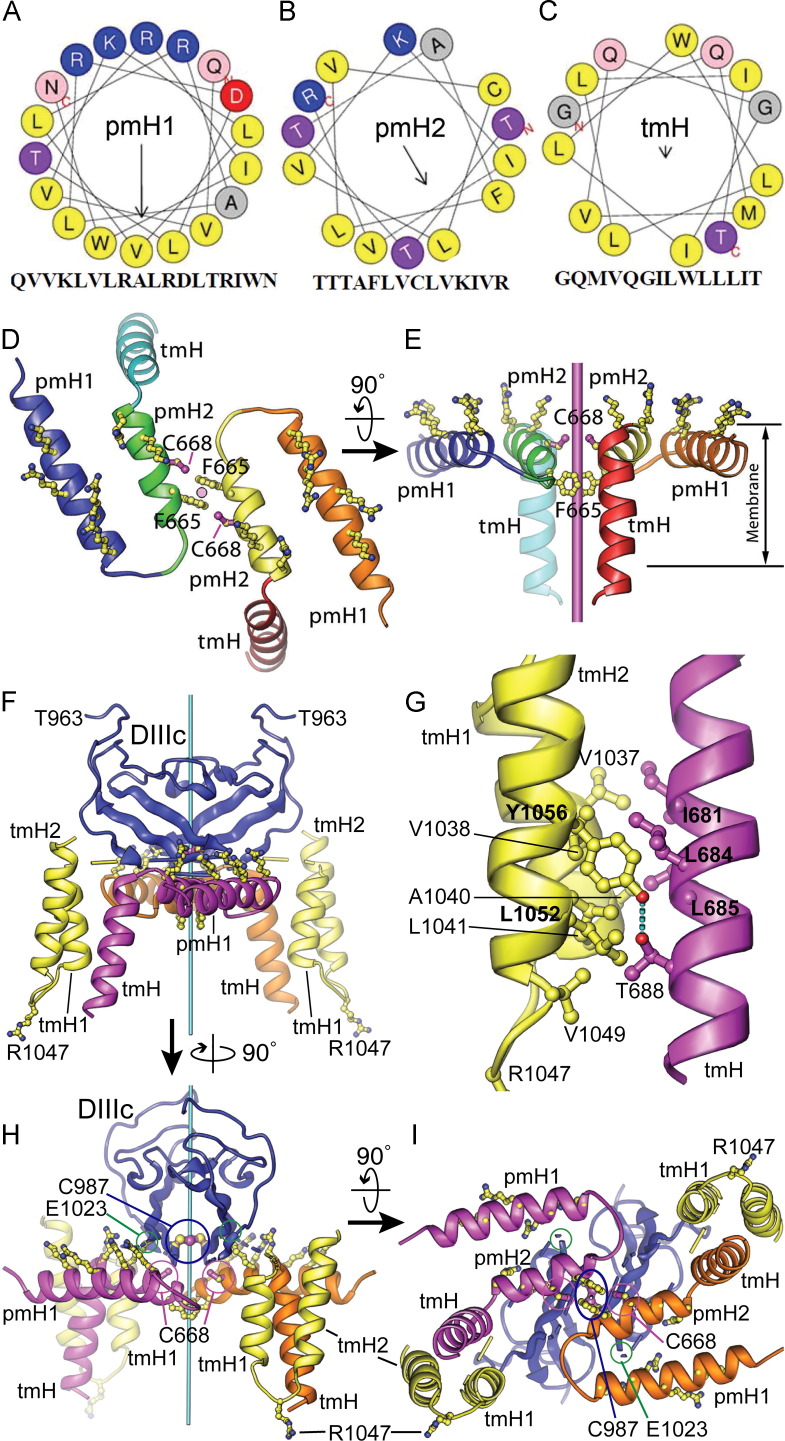
Fig. 4.
Helical wheel modeling of the E1 membrane anchor, and atomic modeling of the E1–E2 oligomeric assembly. (A–C) Helical wheel representations of the core portions of the three membrane-anchored helices in E1: perimembrane helices 1 and 2 (pmH1 and pmH2), and the transmembrane helix (tmH). Interaction of the left side of pmH1 with the right side of pmH2 would position Cys668 (“C”) near the dyad and avoid burying the charged aspartate side chain (“D”) at the interface between the two helices. The arrows represent the direction and magnitude of the amphipathic moment of each helix. (D–E) Orthogonal views of a computational model of two E1 membrane anchors placed to dock optimally on the model of E2 shown in Fig. 2. The dyad is shown in cyan. (F) Computational model of an E1–E2 heterotetramer consistent with the crystallographic E2 homodimer and with the formation of a disulfide bond between E1 C668 and E2 C987. The two E2 fragments are shown in blue (domain IIIc, DIIIc) and yellow (TM helices tmH1, tmH2). The two E1 membrane anchors are in magenta and orange, respectively. The relative position and orientation of DIIIc relative to the membrane anchor assembly may vary depending on the structure of the linker between DIIIc and tmH1 (yellow/blue dashed lines). (G) Close-up of the interface between the E1 tmH and E2 tmH1 helices. A hydrogen bond between E2 Y1056 and E1 T688 stabilizes the otherwise hydrophobic interface. (H-I) Two alternative views of panel F. In the modeled prefusion conformation, the Cα atoms of E1 C668 (cysteine VI, circled in magenta) and E2 C987 (circled in blue) are 6.9 Å apart, within the range for disulfide bond formation.