Figure 1.
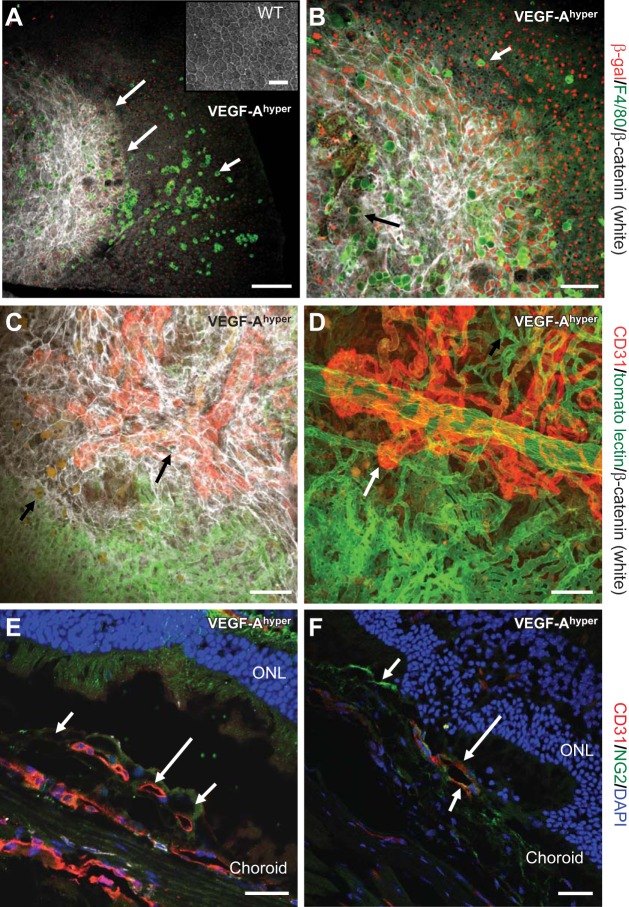
Progressive RPE barrier breakdown and CNV lesion formation in mice with increased VEGF-A levels. A, B) Choroidal flat mount of a 21-mo-old black VEGF-Ahyper mouse. RPE barrier breakdown (indicated as increased β-catenin labeling in white, demarcated by long arrows in A) in VEGF-Ahyper mice is shown with loss of the regular honeycomb RPE cell morphology and by increased cytoplasmic β-catenin labeling (white). In this mouse, almost the entire posterior central fundus shows RPE barrier breakdown with increased β-catenin labeling. F4/80+ macrophages (bright green, short arrow) infiltrate the subretinal space at the site of RPE barrier breakdown. Round autofluorescent sub-RPE deposits (dim green, black arrow in B) are observed at sites of RPE barrier breakdown as well. Red nuclear staining shows labeling for β-galactosidase in these mice, representing VEGF-A expression in pigmented RPE cells (VEGF-Ahyper mice express β-galactosidase from the endogenous VEGF-A locus). Notably, F4/80+ macrophages are β-galactosidase negative. Inset: WT choroidal flat mount with regular honeycomb appearance of RPE cells. C) CNV lesion formation in VEGF-Ahyper mice with neovascularization evolving from choroidal vessels. White VEGF-Ahyper mouse perfused with FITC-tomato lectin and subsequently immunolabeled for CD31 (red) and β-catenin (white). Green vessels represent perfused normal choroidal vasculature, while CD31+ red neovessels originate from the normal choroidal vasculature and form CNV lesions. This CNV lesion formation (red, CD31+vessels, long arrow) occurs at sites of RPE barrier breakdown (white, increased β-catenin). Bottom part of image shows normal choroidal vasculature (green) covered by normal RPE (regular β-catenin labeling); top part shows neovessels (CD31+ in red) covered by irregular RPE cells with increased β-catenin labeling. Autofluorescent sub-RPE deposits appear at sites of RPE barrier breakdown (short arrow). D) Strongly CD31 expressing neovessels (red, long arrow), originating from underlying choroidal vessels (green, FITC-tomato lectin perfusion). White VEGF-Ahyper mouse, age 15 mo, with nonpigmented RPE cells. E) CNV lesions in black VEGF-Ahyper mice protrude into the subretinal space and are CD31+. Frozen section shows CD31+ (red) CNV into the subretinal space (long arrow). Pigmented RPE cells indicated by short arrows. ONL, outer nuclear layer of photoreceptors. F) Vessels in CNV lesions are CD31+ (red, CD31, long arrow) and surrounded in part by NG2+ cells (NG2, green; short arrows). Nuclei labeled blue with DAPI. Black VEGF-Ahyper mouse, age 12 mo. Scale bars = 200 μm (A); 100 μm (B–D); 50 μm (E, F).