Figure 1.
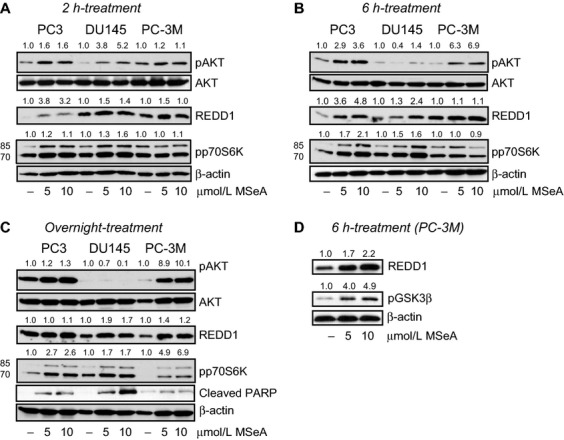
Protein expression changes in cytosolic fractions of PC3, DU145, and PC-3M cells following MSeA treatments for 2 h, 6 h and overnight in hypoxia. The three prostate cancer cell types were stimulated with 10% FBS following an overnight starvation (0.1% FBS) in the presence and absence of MSeA in hypoxia (1% O2). (A) Relatively increased levels of pAKT, REDD1 and pp70S6K (lower band) were observed after 2 h of MSeA treatment in invasive prostate cancer cells in hypoxia compared to respective untreated controls. (B) MSeA treatment after 6 h maintains elevated levels of pAKT in PC3 and PC-3M cells. REDD1 and pp70S6K levels induced by MSeA continue to increase in the three cell types at this time point. (C) An overnight exposure of invasive prostate cancer cells to MSeA maintained high levels of pAKT in PC3 and PC-3M cells while pAKT protein expression was marginally detectable in DU145 cells. The increase in REDD1 levels are more pronounced in the DU145 and PC-3M cells at this time point and pp70S6K expression was relatively higher in MSeA-treated cells as compared to untreated controls in each cell line. In addition, cleaved PARP appeared in a dose-dependent manner in each of the invasive prostate cancer cells following an overnight exposure with MSeA in hypoxia. (D) Following a 6 h treatment with MSeA in PC-3M cells, a concomitant dose-dependent elevation in pGSK3β expression was observed. Fold change in band densities of phosphorylated proteins were normalized to the band densities of their respective native protein and to β-actin levels. While for pp70S6K, pGSK3β and REDD1 the band densities were normalized to β-actin levels. FBS, fetal bovine serum; MSeA, methylseleninic acid; PARP, poly ADP-ribose polymerase; REDD1, regulated in development and DNA damage 1.
