Abstract
Introduction:
The differentiated thyroid cancers have a good prognosis unless the presence of metastasis. These distant metastases, especially in bone, are a major cause of impaired quality of life and death requiring intensive management. The aim of our work was to study the patients' data, the disease characteristics and to analyze the therapeutic management of these patients.
Results:
This study concerned a cohort of 21 patients treated for differentiated thyroid cancer during the period from 1995 to 2011. Eighteen of our patients were aged over 45 years. A majority of them had follicular carcinoma. Bone metastases were often multiple and located at the axial skeleton. They were associated with other types of metastases, especially lung metastasis. A majority of patients received 131I treatment, following surgery or external beam radiotherapy for a palliative purpose. Overall survival was 65% at 5 years and 49% at 10 years. A long-term survival was achieved in 10% of the patients benefiting from a multidisciplinary care adapted to each case.
Conclusion:
Bone metastases often have a pejorative turning in the natural history of differentiated thyroid cancers. The right selection of individuals with better prognosis, for whom more aggressive curative treatment was indicated, requires a better understanding of the features of bone involvement.
Keywords: Bone metastases, differentiated thyroid cancer, iodine-131, thyroglobulin
INTRODUCTION
The differentiated thyroid carcinoma (DTC) is generally considered to be a neoplastic disease having a good prognosis with mortality rates around 5-15%. [1] This is partly due to a spontaneous evolution often slow and to the ability of cancer cells to capture the iodine 131 (131I). Nevertheless, DTC has a significant metastatic potential occurring preferentially in the lung parenchyma, but may also interest the skeleton. These distant metastases, especially in bone, are a major cause of impaired quality of life and cancer deaths. But unlike metastases of other cancers, they are compatible with prolonged survival in some patients. [2] The treatment of bone metastases taking origin from thyroid carcinoma is based on iodine therapy administered as oral periodic cures following total thyroidectomy [3] and is sometimes supplemented by local treatment, surgery, or radiotherapy when secondary lesions became symptomatic. In most cases, this support has palliative purposes.
Recently, many studies tried to develop a more aggressive therapeutic approach, including surgery in some of these patients with curative purpose. [4] In addition to the 131I, these treatments are based on the oncological resection of bone metastases. This type of surgery has, however, a high risk of hemorrhagic complications. This is why the information should be discussed in a multidisciplinary way, case by case, and takes into account prognostic factors in these patients. In addition, this surgery will be done by highly trained surgeons. We studied a cohort of 21 patients with DTC with bone metastases treated and monitored in the nuclear medicine department of Sfax from 1995 to 2011. We studied the characteristics of these patients, their thyroid cancer and secondary bone involvement. We analyzed their therapeutic management.
MATERIALS AND METHODS
Among patients treated for thyroid cancer at Habib Bourguiba Hospital from January 1995 to December 2010 (medullary carcinoma excluded), 21 patients with bone metastases associated or not with other types of distant metastases were included.
A retrospective study was performed. Clinical data were extracted from the medical record, including details on diagnosis, stage, age, site, tumor factors (size and histology), thyroglobulin (Tg) levels, imaging findings, treatment details, response to treatment, disease status, and cause of death. Study end points included date of last follow-up and date of death. Mean time of follow-up was 6.4 years from the diagnosis of thyroid cancer and 3.4 years from the date of bone metastasis presentation.
Distant metastasis at presentation was evaluated using clinical, surgical, and radiographic evidence at the time of diagnosis. Radiographic imaging used included chest X-rays, computed tomography (CT), magnetic resonance imaging (MRI), ultrasound,99m Tc-methylene diphosphonate (99m Tc-MDP) scan, and diagnostic 131I scans. Positron emission tomography (PET) was not available during the time period of the study. Iodine avidity was determined by visual uptake in the known site of metastatic disease revealed on scans, 5 days after the131 I therapy, under hypothyroid conditions. Absence of visual uptake on scan was classified as disease being no iodine avid. All patients were staged in accordance with TNM staging (tumor, node, metastases) system of the UICC/AJCC (Union for International Cancer Control/American Joint Committee on Cancer). [3]
Statistical analysis
Statistical analysis was performed using SPSS 18.0 for windows (Chicago, IL, USA) software. Association between categorical variables was evaluated using a Chi-square test or Fisher's exact test. A P < 0.05 was considered significant. Survival curves were plotted with Kaplan-Meier Method and log-rank test.
RESULTS
Clinical and pathological features are presented in Table 1. There were 7 male and 14 female patients. Mean age at the diagnosis of thyroid cancer was 55 years ± 10.7 years (range: 32-75 years).
Table 1.
Characteristics of patients with bone metastasis resulting from thyroid cancer
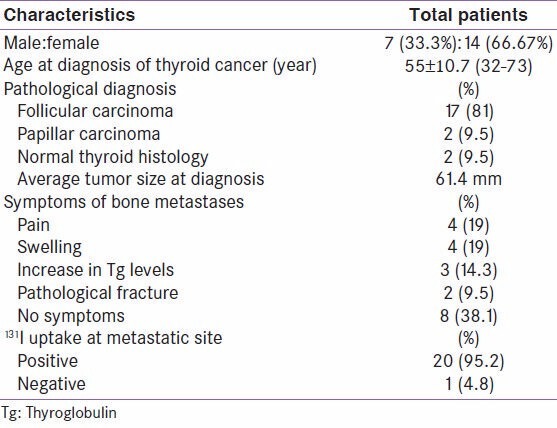
For 17 patients (81%), the histology of primitif tumor was a follicular carcinoma. Papillary carcinoma was found in two cases (9.5%). In two cases, histological examination showed a normal thyroid without histological signs of malignancy. The average tumor size at diagnosis was 61.4 mm (range: 0-120 mm).
Bone metastases were the circumstances of discovery of the primary tumor in 8 patients (38.1%). They appeared during evolution in seven patients (33.3%). They were present at the time of diagnosis of the primary tumor in six patients (28.6%). Clinical signs of bone metastases were pain in four patients (19%), swelling in four patients (19%), an increase in Tg levels in three patients (14.3%), and pathological fracture in two patients (9.5%). Bone metastases were discovered on the post-iodine therapy scan in eight patients without clinical symptoms (38.1%).
The presence of other distant metastases was observed in 18 patients (87%). These metastases are located in the lungs (13 patients), brain (2 patients), eyeball (2 patients), and liver (1 patient) [Table 1].
131I uptake in metastasis was found in 20 patients (95.2%). Bone metastases were multifocal in 76% of cases. They were located successively in descending order of frequency in the limbs, spine, skull, pelvis, sternum, and ribs.
Radiological explorations by standard radiographs, CT, or MRI were performed in 16 patients (76%). They revealed osteolytic metastases for all patients. The remaining five patients had metastases with uptake in the 131I scan or99m Tc-MDP bone scintigraphy and had no radiological explorations.
Treatment of bone metastases
The median cumulative activity administered to patients was 33.3 GBq (900 mCi) (range: 3.7-99.9 GBq; 100-2700 mCi). In our study, we did not note adverse effects related to high doses such as the appearance of secondary cancers or pulmonary fibrosis.
Radiotherapy for bone metastatic sites was performed in 10 patients (47.6%).
Surgery of metastases was performed only in four patients (19%). In two patients, bone metastasis was the circumstance of discovery of the primary tumor. In two patients, the discovery of bone metastases was concomitant to the primary tumor, and surgery of metastases was made at the same time as thyroid surgery.
Evolution and survival analysis
Follow-up was clinical, scintigraphic, and biological. Other radiological examinations were requested as needed.
After a mean time of follow-up of 6.4 years from the diagnosis of thyroid cancer and 3.4 years from the date of bone metastasis presentation, the clinical course of our patients was as follows:
Partial remission was noted only in one patient (4.7%) [Figures 1–3]
A steady state was observed in 9 patients (42.8%)
A clinical worsening was observed in 11 patients (52.3%).
Figure 1.
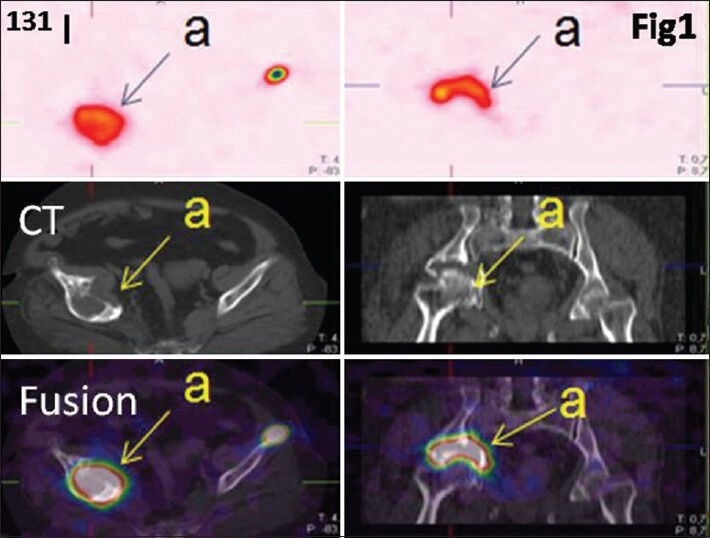
Patient operated for thyroid follicular carcinoma non-metastatic. She presented 13 years later pain in the right hip. A computed tomography objectified bone lysis
Figure 3.
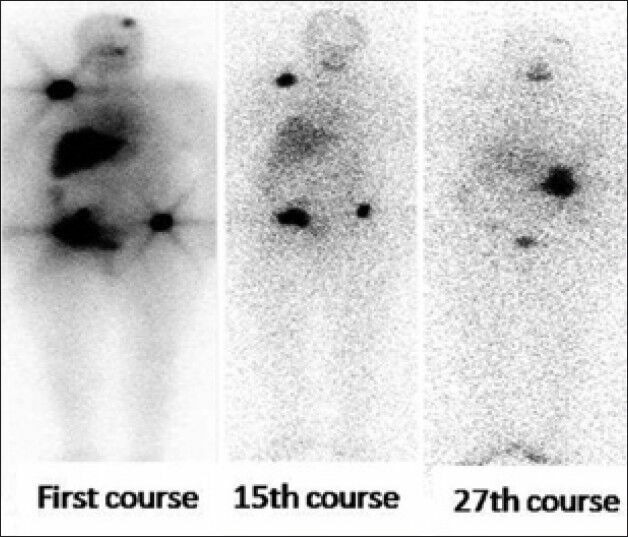
The patient received 27 courses of 131I. Clinical, biological, radiological, and scintigraphic improvements were observed
Figure 2.
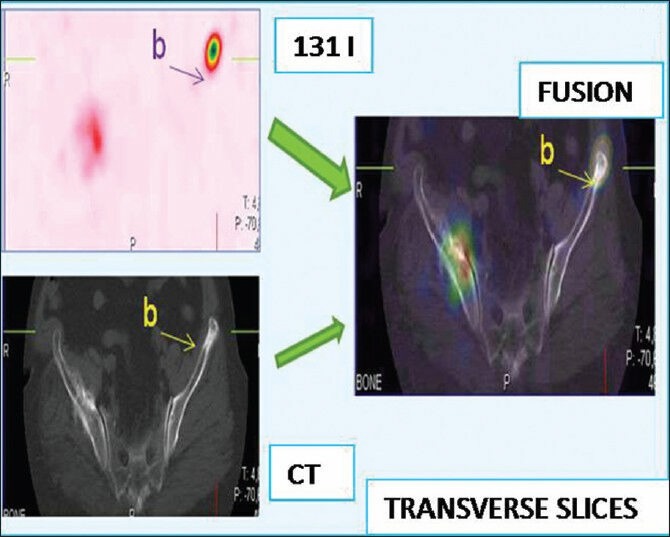
Thyroid origin was confirmed by biopsy. 131I whole body scan finds lesions at the left parietal bone and the right shoulder. Image fusion with computed tomography finds a lysis of the left iliac wing not described
Six of them (28.5%) died of their disease.
Scintigraphic evolution
The evolution of foci uptake on 131I scanning has been specified for 19 patients. For one patient, bone metastases did not fix iodine. The other patient received only one 131I treatment (3.7 GBq), and then she followed and refused to take other courses. The disappearance of foci bone uptake was noted in one patient (5.2%), the reduction in two patients (10.5%), and stabilization in nine patients (47.3%). There was a worsening of bone uptake despite the iodine treatment for seven patients (36.8%).
Tg stimulated levels was reduced to four patients (19%), a stabilization for five patients (23.8%), and an increase in 12 patients (57.2%).
Survival analysis
Survival curves and rates are described in Figure 4.
Figure 4.
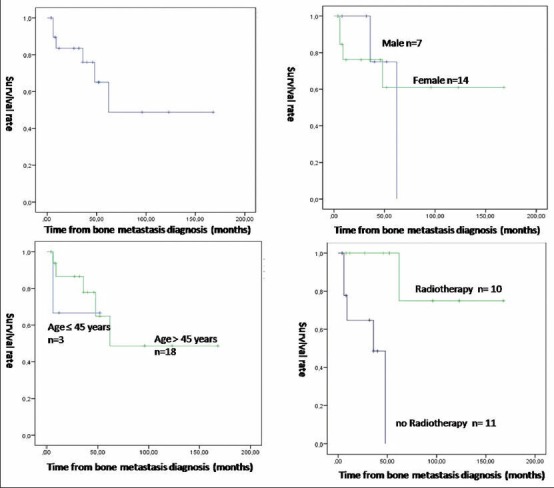
Survival curves using Kaplan-Meier method and Log-rank test. (a) Overall survival curve. (b) Comparison of survival between male and female (P = 0.015). (c) Comparison of survival between patients aged ≤45 years and patients aged >45 years (P = 0.176). (d) Comparison of survival between patients having radiotherapy on metastatic sites and patients who did not (P < 0.001)
Overall survival was 65% at 5 years and 49% at 10 years. Median rate survival was 67.3 months (5.6 years) ranging from 4 months to 336 months (0.3-28 years). Because of the relatively low number of our population, we have been studied only three factors in the survival function: Sex, age, and whether or not radiotherapy was done.
The 5-year survival was 75% for males and 61% for females. A 10-year survival drops to 0% for males and rest 61% for females. The difference between the two groups was significant (P = 0.015). The 5-year survival was 67% for patients ≤45 years and 65% for patients >45 years. The 10-year survival was still at 67% for patients ≤45 years and rose to 48% for patients >45 years. The difference is not significant (P = 0.167).
Finally, survival at 5 years was 0% for patients who did not have radiotherapy. It was 74% for patients who underwent radiotherapy. A 10-year survival was similar for patients who underwent radiotherapy on metastatic sites. The difference is very significant between the two groups (P < 0.05).
DISCUSSION
The DTCs, which represent more than 85% of thyroid cancers, are a small proportion of all cancers, approximately 1.5%. [1] In our region of Sfax, they represent 0.66% of all cancers in men and 3.76% in women. No data are available at national level in Tunisia. Our study is the first study that interested metastatic thyroid cancers in Tunisia.
Among patients with DTC, 17.8-23.5% show a metastatic evolution. [1] If we consider bone disease, they represent only 3.8-17.9% of patients, with an average of 8.9%. [1] In 50-80% of cases, it is a multiple bone lesions. [2,3,4] Our results are concordant with these findings as multiple bone lesions were accounted for 76% of cases.
According to different studies, bone metastases are common in patients with follicular carcinomas (15.2-33.7%) than papillary carcinomas (0.6-6.9%). [5,6,7] Thereby, in patients with DTC with bone metastases, 70% have follicular carcinomas. [5,8] This finding is concordant with our results (follicular carcinoma represents 81% in our study).
The average age at diagnosis of bone metastases is 60 years and 40-50 years for primary thyroid cancer. [3,4,9] In our patient cohort, the mean age at diagnosis for primary cancer was 55 years ± 10.75 years.
Bone metastases occur in DTC under three main circumstances. First, it can be indicative of DTC. It was the case for eight patients in our series (38.1%). This relatively high rate can be explained by the delay in the consultation in our population.
Second, metastatic bone disease may be discovered during the staging of patients treated for differentiated thyroid cancer. In these cases, scans with 131I may reveal uptake in the skeleton affirming the thyroid origin of metastases. This mode of revelation was seen in six patients (28.6%). Finally, as seen in 7 patients (33.3%), metastasis may be discovered after the onset of symptoms suggestive of bone metastasis during follow-up or after an elevation of Tg level.
The most common manifestations of bone metastases of DTC include pain, fractures, and spinal cord compression associated with lesions in the axial skeleton.
These symptoms are sometimes due to metastatic complication, especially pathological fractures that occur in 20-30% of patients with pain and functional impairment according to the degree of severity of bone involvement. [9]
These complications may also include neurological damages such as spinal nerve root compression (14-23.8%). [9]
The spread of metastases may occur in any segment of bone. However, in the literature, there is a preferential involvement of the axial skeleton, particularly the spine, the pelvis, and ribs. [9] This was found in our population.
The bone lesions in patients with DTC are mostly osteolytic. This finding is in line with our results (16 patients).
Bone scan with injection of99m Tc bisphosphonates is rarely used in the context of DTC because it can give false-negative results. [10,11]
Single photon emitted computer tomography coupled with CT combine the advantages of high sensitivity imaging of bone metabolism with those of a high-resolution imaging of bone morphology. It improves the sensitivity and specificity especially of planar bone scintigraphy.
Whole body scans post-131I therapy are both more specific and more sensitive than99m Tc-MDP in the detection of bone metastases, but only for well differentiated, NIS-positive thyroid tumors. [12,13] However, the presence of multiple bone lesions correlates with aggressive tumors which are usually less differentiated. PET scan seems to be interesting in these cases.
Bone metastasis treatment
Bone metastases tend to be highly vascular. This highlights the need for special techniques to avoid excessive perioperative mortality. Affected patients should be referred to a multidisciplinary management team for an optimal care. The general management principles are the same as for non-metastatic thyroid cancer. Most often, the dose is 100 mCi (3.7 GBq) or 150 mCi (5.55 GBq) of 131I. The objective of treatment is to achieve sufficient concentration of radioactive iodine in tumor areas for treatment efficacy. [14] This efficiency will be even more pronounced than the volume of each tumor is reduced, even if there are multiple lesions.
Data from literature show that 40-88.3% of patients with bone metastases receive radioiodine for therapy. Cumulative average doses administered ranged from 319 ± 281 mCi to 680 mCi. [2,9] The mean cumulative dose in this study was 900 mCi with the highest dose of 3Ci administered to one patient with favorable clinical, biological, and imaging evolution. No side effects related to high dose of iodine, such secondary cancers or pulmonary fibrosis, were noted in our patients. External beam radiotherapy is primarily indicated for analgesic purpose. This was the case in our patients. It may be indicated in some cases of neurological compression.
Prognosis
Several studies have previously been conducted to evaluate the factors affecting the DTC prognosis. Differences in results may be attributed to population characteristics, the factors taken into account, and treatment used. [15,16,17,18] Based on available data, the only treatment option is usually palliative care in patients with DTC, although some teams are starting to offer more aggressive treatment for curative purposes.
Females have a better prognosis. In our study, we observe a significant difference between males and females in terms of the survival analysis. The difference was significant for the 10-year survival. No difference in survival at 5 years was found. A study by Mazzaferri describes the female gender as a good prognostic factor for metastatic patients. [19]
Several studies have shown that patients ≤45 years at diagnosis of their bone metastases have a significantly better prognosis than those >45 years. This difference appeared to be significant in univariate analysis for both studies, [2] and multivariate analysis for three studies. [20,21]
We can hypothesize that carcinomas occurring in older patients are more aggressive and therefore more likely to cause a metastatic evolution. In our study, we did not find any significant difference in survival analysis due to lack of power (reduced number of the population).
Treatment with iodine-131 appears to be a significant prognostic factor associated with better survival. In the literature, the results of different studies are contradictory. If for Bernier and Pittas, it is a positive prognostic factor. [2,9] Dinneen shows no improvement in survival of patients receiving iodine-131[34]. However, the study of Petrich et al. described a distinct advantage for patients treated with iodine-131, much more if they are young (<45 years) and carrying a small number of metastatic sites (<3) with higher complete response rate. [22]
For radiotherapy, the study of Bernier et al. [2] showed no improvement in survival associated with external radiotherapy of bone metastases. But it was limited doses adapted to a consolidator and analgesic treatment not for curative purposes. However, it is important to implement with patterns adapted to the condition and prognosis of patients to improve pain symptoms.
In our study, we find a significant difference in terms of survival. Further studies including more patients are necessary to confirm this result.
CONCLUSION
Bone metastasis has often a pejorative turning in the natural history of differentiated cancers of the thyroid. Treatment with radioactive iodine alone cannot give good results in terms of clinical outcome and survival. At best, it provides stabilization of lesions. External radiotherapy and surgery should be indicated to improve the prognosis of these patients. Currently, several other treatments such as anti-angiogenic gene therapy are being evaluated. We believe that the development of a prognostic score for patients with bone metastases could allow the selection of individuals with better prognosis for whom more aggressive curative treatment would be indicated.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Schlumberger Martin, Pacini F. In: Epidemiology. Nucléon, editor. Paris: Thyroid Tumors; 1999. pp. 47–60. [Google Scholar]
- 2.Bernier MO, Leenhardt L, Hoang C, Aurengo A, Mary JY, Menegaux F, et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86:1568–73. doi: 10.1210/jcem.86.4.7390. [DOI] [PubMed] [Google Scholar]
- 3.Stratmann M, Sekulla C, Dralle H, Brauckhoff M. Current TNM system of the UICC/AJCC: The prognostic significance for differentiated thyroid carcinoma. Chirurg. 2012;83:646–51. doi: 10.1007/s00104-011-2216-3. [DOI] [PubMed] [Google Scholar]
- 4.Fanchiang JK, Lin JD, Huang MJ, Shih HN. Papillary and follicular thyroid carcinomas with bone metastases: A series of 39 cases during a period of 18 years. Changgeng Yi Xue Za Zhi. 1998;21:377–82. [PubMed] [Google Scholar]
- 5.Lin JD, Liou MJ, Chao TC, Weng HF, Ho YS. Prognostic variables of papillary and follicular thyroid carcinoma patients with lymph node metastases and without distant metastases. Endocr Relat Cancer. 1999;6:109–15. doi: 10.1677/erc.0.0060109. [DOI] [PubMed] [Google Scholar]
- 6.Muresan MM, Olivier P, Leclère J, Sirveaux F, Brunaud L, Klein M, et al. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. 2008;15:37–49. doi: 10.1677/ERC-07-0229. [DOI] [PubMed] [Google Scholar]
- 7.Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. 2007;110:1451–6. doi: 10.1002/cncr.22956. [DOI] [PubMed] [Google Scholar]
- 8.Tickoo SK, Pittas AG, Adler M, Fazzari M, Larson SM, Robbins RJ, et al. Bone metastases from thyroid carcinoma: A histopathologic study with clinical correlates. Arch Pathol Lab Med. 2000;124:1440–7. doi: 10.5858/2000-124-1440-BMFTC. [DOI] [PubMed] [Google Scholar]
- 9.Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, et al. Bone metastases from thyroid carcinoma: Clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10:261–8. doi: 10.1089/thy.2000.10.261. [DOI] [PubMed] [Google Scholar]
- 10.Feine U. Fluor-18-deoxyglucose positron emission tomography in differentiated thyroid cancer. Eur J Endocrinol. 1998;138:492–6. doi: 10.1530/eje.0.1380492. [DOI] [PubMed] [Google Scholar]
- 11.Ito S, Kato K, Ikeda M, Iwano S, Makino N, Tadokoro M, et al. Comparison of 18F-FDG PET and bone scintigraphy in detection of bone metastases of thyroid cancer. J Nucl Med. 2007;48:889–95. doi: 10.2967/jnumed.106.039479. [DOI] [PubMed] [Google Scholar]
- 12.Schirrmeister H, Buck A, Guhlmann A, Reske SN. Anatomical distribution and sclerotic activity of bone metastases from thyroid cancer assessed with F-18 sodium fluoride positron emission tomography. Thyroid. 2001;11:677–83. doi: 10.1089/105072501750362754. [DOI] [PubMed] [Google Scholar]
- 13.de Geus-Oei LF, Oei HY, Hennemann G, Krenning EP. Sensitivity of 123I whole-body scan and thyroglobulin in the detection of metastases or recurrent differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2002;29:768–74. doi: 10.1007/s00259-002-0781-x. [DOI] [PubMed] [Google Scholar]
- 14.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 15.Schlumberger M, Pacini F. In: Thyroid Tumors. Nucléon, editor. Paris: 1999. pp. 33–46. [Google Scholar]
- 16.Do MY, Rhee Y, Kim DJ, Kim CS, Nam KH, Ahn CW, et al. Clinical features of bone metastases resulting from thyroid cancer: A review of 28 patients over a 20-year period. Endocr J. 2005;52:701–7. doi: 10.1507/endocrj.52.701. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: Analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36:1274–8. doi: 10.1007/s00268-012-1423-5. [DOI] [PubMed] [Google Scholar]
- 18.Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. 2003;197:191–7. doi: 10.1016/S1072-7515(03)00332-6. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferri EL, Young RL. Papillary thyroid carcinoma: A 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511–8. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 20.Casara D, Rubello D, Saladini G, Gallo V, Masarotto G, Busnardo B. Distant metastases in differentiated thyroid cancer: Long-term results of radioiodine treatment and statistical analysis of prognostic factors in 214 patients. Tumori. 1991;77:432–6. doi: 10.1177/030089169107700512. [DOI] [PubMed] [Google Scholar]
- 21.Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID. Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995;80:2041–5. doi: 10.1210/jcem.80.7.7608252. [DOI] [PubMed] [Google Scholar]
- 22.Petrich T, Widjaja A, Musholt TJ, Hofmann M, Brunkhorst T, Ehrenheim C, et al. Outcome after radioiodine therapy in 107 patients with differentiated thyroid carcinoma and initial bone metastases: Side-effects and influence of age. Eur J Nucl Med. 2001;28:203–8. doi: 10.1007/s002590000420. [DOI] [PubMed] [Google Scholar]


