Fig. 1.
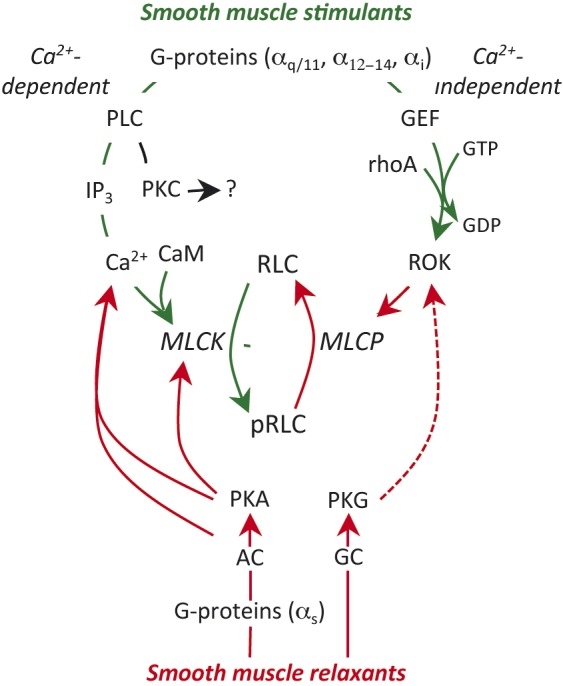
Regulation of smooth muscle contractility. Uterine stimulants generally stimulate specific G-protein coupled receptors (GPCR) on the myocyte membrane. This triggers two stimulatory pathways. The Gαq subunit activates phospholipase C (PLC) in the adjacent membrane and this results in hydrolysis of membrane phosphoinositides to from inositol trisphosphate (IP3) and diacyl glycerol, which can activate protein kinase C (PKC; see text). IP3 stimulates release of Ca2+ from the sarcoplasmic reticulum and the resulting decrease in cell membrane resting potential leads to massive influx of Ca2+ from the extracellular space through voltage–gated L-type Ca2+-channels. Ca2+ binds to calmodulin (CaM). The Ca2+-CaM complex interacts with and activates myosin regulatory light chain kinase (MLCK). MLCK mediates the phosphorylation of myosin regulatory lights chains (RLC) and initiates the contractile apparatus. The second stimulatory pathway is mediated through Gα12-14. This causes activation of a guanidine nucleotide exchange factor (GEF), which activates membrane rhoA-GDP by substituting GTP for the GDP. The rhoA-GTP activates rhoA-associated kinase (ROK). ROK phosphorylates myosin regulatory light chain phosphatase (MLCP), which inhibits its activity. Since MLCP is normally responsible for removing the phosphate from pRLC to cause uterine relaxation, enhanced ROK activity prolongs the effectiveness of pRLC and promotes enhanced contractile activity. The major inhibitory pathways might also be mediated by GPCR, linked through Gαs resulting in activation of adenylate cyclase (AC) or direct activation of guanylate cyclase (GC), which in turn activates protein kinase A (PKA) or PKG. These kinases inhibit contractility through a variety of mechanisms described in the text. The green lines indicate stimulatory activity and the red lines inhibitory. The broken line indicates an indirect effect.
