Abstract
Malaria and dengue fever are major mosquito-borne public health problems in tropical countries. The authors report a malaria and dengue co-infection in an 11-year-old boy who presented with sustained fever for 10 days. The physical examination revealed a flushed face, injected conjunctivae and left submandibular lymphadenopathy. His peripheral blood smear showed few ring-form trophozoites of Plasmodium falciparum. His blood tests were positive for dengue NS-1 antigen and IgM antibody, and negative for IgG antibody. After the initiation of antimalarial treatment with artesunate and mefloquine, his clinical condition gradually improved. However, he still had low-grade fever that persisted for 6 days. Finally, he recovered well without fluid leakage, shock or severe bleeding. This case report emphasises that early recognition and concomitant treatment of malaria and dengue co-infection in endemic areas can improve clinical outcome and prevent serious complications.
Background
Thailand is an endemic area for many tropical infectious diseases, including malaria and dengue fever. Both of them present not only a significant mosquito-borne public health problem but also share similar clinical presentations, including fever and thrombocytopenia. However, co-infections of dengue and malaria are not common in our clinical practice, and few case reports of co-infections from countries where both diseases are endemic have been published. Most case reports are from Africa,1 Brazil2 and India3–5 involving adult patients. One study from East Timor in 2006 reported a co-infection with Plasmodium falciparum malaria and dengue in a 7-year-old girl who subsequently died.6 Three further descriptive studies and one case control study were published from India7 8 and French Guiana,9 10 all patients in these studies being adult patients. Malaria and dengue co-infections were found in only 11 (10.6%) of 104 co-infected paediatric patients from French Guiana between 2004 and 2010.10 A Thai study on co-infections in 194 adult malaria patients in 2006 reported malaria co-infections with murine typhus (23.2%), scrub typhus (15%) and leptospirosis (7.7%), but did not report any malaria and dengue co-infections.11 An earlier Thai study on co-infections in paediatric dengue patients from 1984 to 1995 reported a co-infection incidence rate of 1:200, and noted melioidosis, Salmonella, Shigella and E coli septicaemia to be the most common co-infections, but did not report any malaria and dengue co-infections either.12 Physicians should recognise co-infections so that they can provide prompt treatment which will lead to better outcomes.
Case presentation
An 11-year-old boy from the northern part of Thailand near the Thai-Burmese border was admitted to the Chiang Mai University Hospital (CMUH) due to sustained fever for 10 days prior to admission. He presented with fever and chills, but had no symptoms of cough, running nose or other respiratory tract symptoms. His appetite was normal and he also had no jaundice, vomiting or diarrhoea. The boy had no history of underlying diseases and his birth history was unremarkable. He had no known drug allergies and his vaccination history was complete. He had a history of unknown partial treatments from a rural Burmese hospital 1 week prior to moving to Thailand. His father remembered that the boy had been given some artesunate tablets without improvement of his condition. Three days before admission to the CMUH, he developed stomach-ache and had several episodes of vomiting. He also complained about myalgia, but did not have any petechiaes or haemorrhage.
On admission, his physical examination revealed a high-grade fever (39.1°C), heart rate 140 bpm and respiratory rate 30 breaths/minute. He looked sick and had a flushed face. He had no pallor, but mildly injected conjunctivae and pharynx, mildly enlarged tonsils and left submandibular lymphadenopathy. His breath sound was normal, but he had tachycardia. He had mild tenderness on the upper right side of the abdomen and epigastrium, without abdominal distension, guarding or rebound tenderness. His liver was mildly enlarged with firm and sharp margins without tenderness. His spleen was normal. There were no petechiae or haemorrhages on his skin. He had drowsiness but good orientations to time, place and person. No neurological deficits were found during admission.
Investigations
Laboratory studies showed a haemoglobin concentration of 12.2 g/dL, a haematocrit 38.0% and a white cell count 11 810 cells/mm3 with 70% neutrophils, 11.2% lymphocytes and 13.7% monocytes. The platelet count was 383 000 cells/mm3. His peripheral blood smear showed normochromic red blood cells with several target cells and few basophilic stipplings, and few ring-form trophozoites of P falciparum in normal-sized red cells (figure 1).
Figure 1.
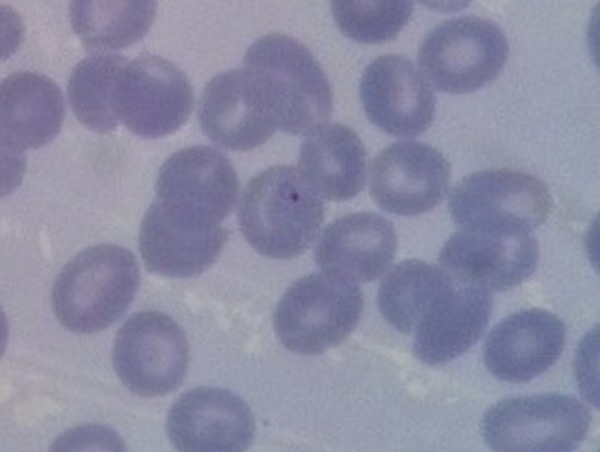
Peripheral blood smear showing ring-form trophozoites of Plasmodium falciparum in normal-sized red cells.
A liver function test revealed a total protein of 8.1 g/dL, albumin 4.4 g/dL, globulin 3.6 g/dL, alkaline phosphatase 229 U/L, cholesterol 118 mg/dL, aspartate aminotransferase 35 U/L, alanine transaminase 32 U/L, total bilirubin 0.53 mg/dL and direct bilirubin 0.09 mg/dL. His blood glucose was 99 mg/dL. His blood chemistry levels and urinary analysis were normal. Anti-HIV was negative. His erythrocyte sedimentation rate was 25 mm/h and C reactive protein level was below 3.3 mg/L.
Dengue NS-1 antigen and IgM antibody were positive, while IgG antibody was negative. A test for leptospirosis IgM and IgG was negative. His glucose-6-phosphate dehydrogenase level was normal. His blood cultures revealed no growth in 5 days. Because he looked sick and was drowsy on admission, we also performed a lumbar puncture. His cerebrospinal fluid profiles were normal. A chest radiograph was unremarkable.
Differential diagnosis
P falciparum malaria and dengue co-infection.
Treatment
The patient received artesunate as an antimalarial medication intravenously due to poor oral intake for 4 days. Then artesunate was switched to an oral form on the fifth day and was continued for 1 week. He also received mefloquine on the second day of admission, and primaquine to kill gametocytes before he was discharged from the hospital. Owing to poor oral intake, he received a normal saline infusion for hydration for 7 days. He was monitored every day for haemoconcentration and thrombocytopenia.
Outcome and follow-up
After the initiation of antimalarial treatment, the patient's clinical outcome improved. His appetite returned to normal in 1 week. There were no complications from malarial infection, such as cerebral malaria, hyperpyrexia, severe anaemia, hypoglycaemia, metabolic acidosis and seizure in this boy. His peripheral blood smears 12 h after starting the treatment did not contain any ring-form trophozoites any more. However, he still had low-grade fever 6 days after admission. We assumed that this persistent fever could be the result of the febrile stage of the dengue infection, which was longer than usual due to the co-infection with malaria. He was also monitored daily for haemoconcentration and thrombocytopenia. His fever gradually resolved at the end of the first week of admission. He had no signs and symptoms of fluid leakage and shock. Although his lowest platelet concentration was 68 000 cells/mm3 on the sixth day of admission, he had only minor epistaxis and no serious bleeding complications occurred. His clinical outcomes returned to normal in the convalescent stage. The boy was discharged after admission to the hospital for 8 days.
Discussion
This 11-year-old boy presented with a P falciparum malaria and dengue co-infection. Malaria and dengue infections are still major mosquito-borne public health problems in tropical regions worldwide. This is the first case report of malaria and dengue co-infection reported in Thailand.11 12 In this case, we recognised this co-infection for three reasons. First, this boy had received effective drugs for malaria treatment (artesunate pills) at a Burmese hospital prior to moving to Thailand, but in spite of this, his fever still persisted. Second, there was a dengue outbreak during the time this boy was admitted to the hospital in our country. Last, his physical examination revealed high-grade fever with a flushed face and mild hepatomegaly, even though malaria patients usually have anaemia. We therefore performed early investigations for both diseases. The swift recognition of the malaria and dengue co-infection in our case would have positively affected treatment outcomes, in contrast to the case from East Timor6 whose diagnosis was delayed and who subsequently died. Our good treatment outcomes could also be the result of having received early effective treatment for malaria and his primary dengue infection which usually had mild clinical symptoms.
A retrospective matched pair study in French Guiana aimed to differentiate how the clinical manifestations of co-infections differ from that of single infections and to determine whether conditions of patients who were infected by both malaria and dengue were more severe than those who were infected with either malaria or dengue alone.10 The authors concluded that dengue and malaria co-infections tended to be more severe than single infections, notably for haematological abnormalities, such as thrombocytopenia (platelet concentration <50 000 cells/mm3) and anaemia (haematocrit <36%). However, we could observe neither severe thrombocytopenia nor anaemia in our case, which could be due to the early recognition and treatment of both diseases.
In a case series from Karachi in Pakistan, it was concluded that the diagnosis of a co-infection could be made on the basis of the patient history and clinical examination supported by haematological results. It was also recommended that all patients suspected to have a co-infection be treated concomitantly for dengue and malaria in malaria endemic areas.8 In our case, we also treated the patient for both co-infection of malaria and dengue, and he was subsequently cured.
Learning points.
All physicians should be able to diagnose malaria and dengue co-infection in patients, especially paediatric patients who live in or have recently returned from areas where both diseases are endemic.
When physicians suspect the presence of a malaria and dengue co-infection, they should promptly treat the patients concomitantly for both diseases and closely monitor them for further complications.
Having a good understanding of the natural histories and clinical symptoms of both diseases will help physicians to recognise the diseases early and predict the clinical outcomes/prognoses of such conditions.
Acknowledgments
The authors would like to thank Albert L Oberdorfer from the English Department, Faculty of Humanities, Chiang Mai University for his editorial work.
Footnotes
Contributors: IS wrote the proposal, collected the data, wrote the manuscript and also read the final draft. WA wrote and edited the manuscript and also read the final draft. OP wrote the proposal and the manuscript, supervised the overall work and read the final draft of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Charrel RN, Brouqui P, Foucault C, et al. Concurrent dengue and malaria. Emerg Infect Dis 2005;11:1153–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santana Vdos S, Lavezzo LC, Mondini A, et al. Concurrent dengue and malaria in the Amazon region. Rev Soc Bras Med Trop 2010;43:508–11 [DOI] [PubMed] [Google Scholar]
- 3.Kaushik RM, Varma A, Kaushik R, et al. Concurrent dengue and malaria due to Plasmodium falciparum and P. vivax. Trans R Soc Trop Med Hyg 2007;101:1048–50 [DOI] [PubMed] [Google Scholar]
- 4.Alam A, Dm M. A case of cerebral malaria and dengue concurrent infection. Asian Pac J Trop Biomed 2013;3:416–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mushtaq MB, Qadri MI, Rashid A. Concurrent infection with dengue and malaria: an unusual presentation. Case Rep Med 2013;2013:520181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward DI. A case of fatal Plasmodium falciparum malaria complicated by acute dengue fever in East Timor. Am J Trop Med Hyg 2006;75:182–5 [PubMed] [Google Scholar]
- 7.Hati AK, Bhattacharjee I, Mukherjee H, et al. Concurrent dengue and malaria in an area in Kolkata. Asian Pac J Trop Med 2012;5:315–17 [DOI] [PubMed] [Google Scholar]
- 8.Abbasi A, Butt N, Sheikh QH, et al. Clinical features, diagnostic techniques and management of dual dengue and malaria infection. J Coll Physicians Surg Pak 2009;19:25–9 [PubMed] [Google Scholar]
- 9.Carme B, Matheus S, Donutil G, et al. Concurrent dengue and malaria in Cayenne Hospital, French Guiana. Emerg Infect Dis 2009;15:668–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epelboin L, Hanf M, Dussart P, et al. Is dengue and malaria co-infection more severe than single infections? A retrospective matched-pair study in French Guiana. Malar J 2012;11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhsilarak T, Phongtananant S, Jenjittikul M, et al. Possible acute coinfections in Thai malaria patients. Southeast Asian J Trop Med Public Health 2006;37:1–4 [PubMed] [Google Scholar]
- 12.Pancharoen C, Thisyakorn U. Co-infection in dengue patients. Pediatr Infect Dis J 1998;17:81–2 [DOI] [PubMed] [Google Scholar]


