Abstract
We report a case of acute (24 h) double flexor tendon rupture of the little finger after a single injection of collagenase clostridium histolyticum into a palmar Dupuytren's contracture cord which caused metacarpophalangeal joint contracture. Tendon surgery was performed 48 h postinjury with primary repair and standard rehabilitation but it resulted in poor active flexion due to adhesions. Previous papers have suggested that a needle inserted into the flexor tendon can be detected prior to the injection of collagenase by asking the patient to actively move the finger, but our test on an awake patient showed that when a 27-gauge needle was inserted into the flexor tendons through a thick palmar cord, the syringe did not move significantly when the patient moved the finger, and therefore this test does not minimise the risk of iatrogenic tendon injury when using collagenase (Xiapex) for Dupuytren's contracture.
Background
Dupuytren's contracture is a very common benign fibro-proliferative disorder of the palmar fascia of the hands that leads to contractures of the metacarpophalangeal (MCP) and the proximal interphalangeal (PIP) joints in the hand making it difficult for patients to perform activities of daily living.
The standard treatment is complex hand surgery, often requiring a general anaesthetic, and requires prolonged postsurgical rehabilitation.1 However, last year, a chemical treatment (collagenase clostridium histolyticum (CCH); Xiapex, Pfizer) administered under local anaesthetic was released for use in the UK which opened up the possibility for this drug to be used in general practice. Although tendon ruptures have been reported, these have only occurred after multiple injections into the finger, suggesting that this is a safe treatment for injection into palmar cords. However, CCH is currently marked with a black triangle in the British National Formulary, which means it is a new compound for which all adverse drug reactions should be recorded.
It has been suggested that a needle inserted into the flexor tendon can be detected prior to the injection of collagenase by asking the patient to actively move the finger and see if the needle moves with the finger.
Questions to answer:
1. Should this drug be used in general practice to treat Dupuytren's contracture?
2. Is this drug safe when used in the palm?
3. Can a clinical test detect placement of the needle in the flexor tendon prior to injection in patients with Dupuytren's contracture?
Case presentation
A 47-year-old right-handed man was referred to our hand clinic via the GP's surgery for bilateral Dupuytren's contracture of the MCP and the PIP joints. He also had left foot plantar fibromatosis and a positive family history (father) of Dupuytren's disease. He was otherwise a non-smoker, did not report any other medical problem and was not on any medication.
The patient presented with bilateral hand deformity that started when he was a teenager; this got progressively worse over the years causing him difficulty in holding objects in his hands. On examination, his right hand was worse and had involvement of little, ring and middle fingers with both MCP and PIP joint involvement. His left hand had palpable cords over little, ring and middle fingers but the contractures were limited to the ring and little fingers with MCP joint involvement only. The deformity was measured as 45° in both MCP joints.
After discussion of the surgical versus CCH injection procedures, the patient opted to try the injection for left hand MCP joint contractures in the first instance.
This was performed after written informed consent. The procedure was undertaken by the senior author. The total quantity of the injection (0.58 mg) was distributed equally between the ring and little finger MCP joint cords in the palm.
The patient was called back after 48 h for manipulation of the contractures. The patient presented for manipulation with a 24 h history (ie, 24 h after the injection) of feeling a snap in the left little finger after he merely brushed it against an empty dinner plate. After this episode, the patient was unable to move this finger.
Clinical examination revealed flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP) rupture of the left little finger.
Investigations
The patient was taken to the operating theatre the next day. Although the overlying skin of the palm looked normal (figure 1), intraoperatively the FDS and FDP to the left little finger were found to be ruptured with extensive haemorrhage in the surrounding tissues at the site of the A1 pulley (figure 2). The neurovascular bundle was found to be intact.
Figure 1.
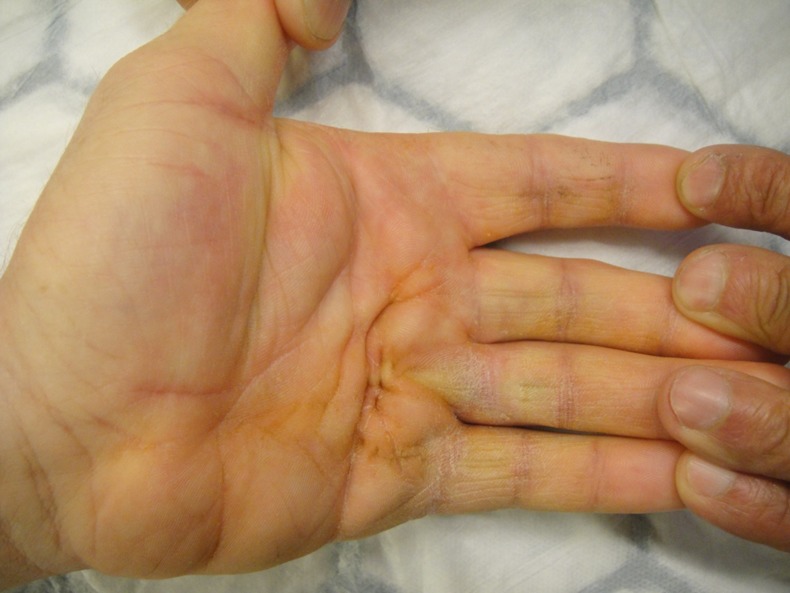
Intact skin of the palm.
Figure 2.
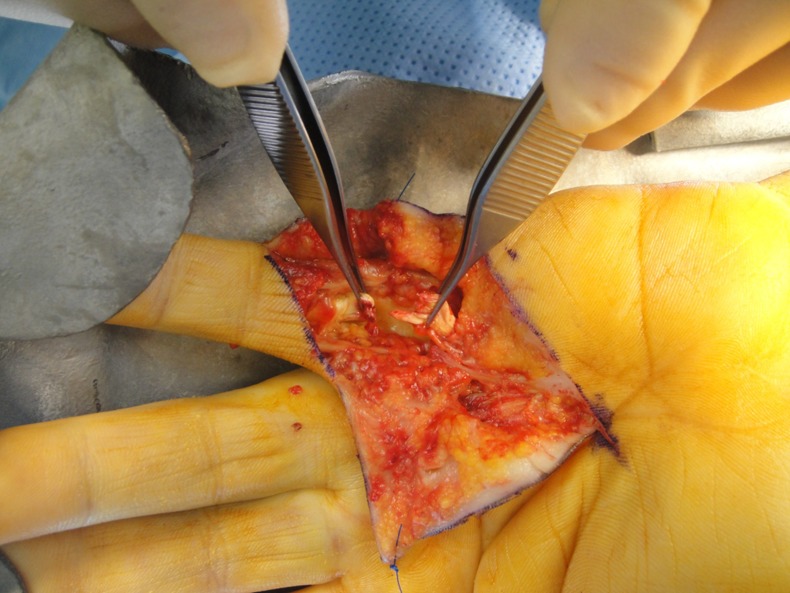
Subcutaneous tendon ruptures and haemorrhage.
Treatment
The tendons were repaired and standard rehabilitation for flexor tendon injuries was provided.
Outcome and follow-up
As the tendon injury had occurred within the past 24 h, we chose to perform a primary repair and rehabilitated the patient according to our standard hand therapist lead rehabilitation programme. However, the active range of movement was disappointing and it may be better in the future to perform a two-stage flexor tendon reconstruction even if the rupture is noticed acutely due to the significant haemorrhage found below the intact skin to minimise tendon adhesions.
Discussion
Rupture of one or both flexor tendons with CCH injection is rare with recent literature citing a figure of 0.5%;2 this compares favourably with open surgical procedures, which report an incidence of 0.2%.3 The ruptures that have been reported so far have been after an injection of PIP joint cord or after multiple injections.
Our case was of an FDP and FDS rupture post a single injection to a palm cord for MCP joint contracture. Though this rupture occurred acutely in our case, the intraoperative findings were similar to those reported by Zhang et al2 where the rupture presented several weeks after the injection with normal overlying skin but extensive surrounding haemorrhage and some loss of tendon substance once the skin was incised. Likewise, the neurovascular bundles were found to be intact. As the tendon injury had occurred within the past 24 h, we chose to perform a primary repair and rehabilitated the patient according to our standard hand therapist lead rehabilitation programme. However, the active range of movement was disappointing and it may be better in the future to perform a two-stage flexor tendon reconstruction even if the rupture is noticed acutely due to the significant haemorrhage found below the intact skin to minimise tendon adhesions.
To the best of our knowledge, this is the first case reporting flexor tendon rupture after a single CCH injection into the palmar cord for Dupuytren MCP joint contracture. This is particularly noteworthy as the dose was divided equally between two cords so the rupture occurred with half the regular dose and attempts were made to inject superficial to the flexor tendon sheath.
Knowing that the flexor tendon averages 7 mm at the MCP joint and 4 mm at the PIP joint and that the bevel of a standard 27-gauge needle is approximately 1.3 mm, we used this knowledge in positioning the needle away from the flexor tendons and holding the syringe before pushing the plunger so that the needle tip did not migrate into the tendon as suggested by Zhan et al2 though on this occasion it did not prevent this complication.
We, therefore, hypothesised that if the needle was inserted into the flexor tendon, this could be detected prior to the injection of collagenase by asking the patient to move the flexor tendon/finger and observe if the needle moved. However, our test on an awake patient showed that when a 27-gauge needle was inserted into the flexor tendons through a thick palmar cord (figure 3), the syringe did not move significantly when the patient moved the finger (figure 4), and we are therefore of the opinion that this test does not remove the risk of iatrogenic tendon injury when using CCH (Xiapex) injection for Dupuytren contracture.
Figure 3.
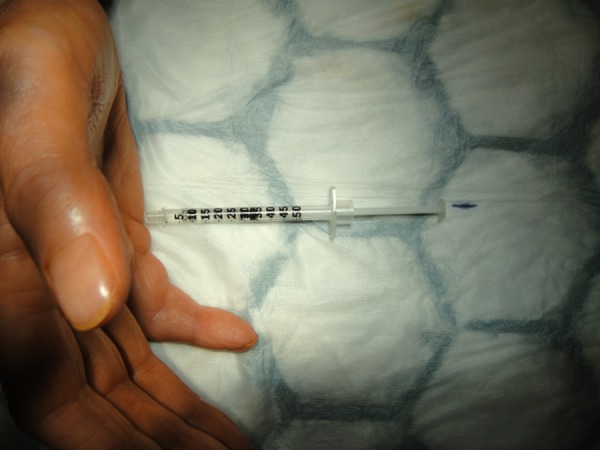
Needle inserted into the flexor tendons through a thick palmar cord.
Figure 4.
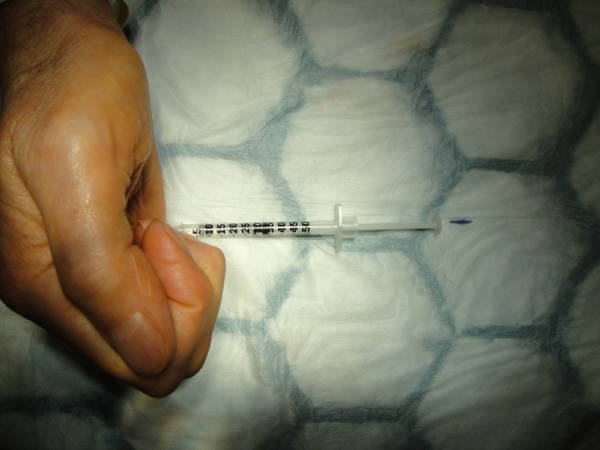
Syringe static on active finger movement.
Learning points.
Collagenase clostridium histolyticum injected into the palm of patients with Dupuytren's contracture can cause tendon rupture.
Clinical test cannot detect if the needle is inserted into the tendon.
Use of collagenase clostridium histolyticum for patients with Dupuytren's contracture in general practice is ill advised due to the risk of tendon rupture.
Current literature which suggests that it is safe to inject collagenase clostridium histolyticum into the palmar cords of Dupuytren's contracture should be revised.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Becker GW, Davis TR. The outcome of surgical treatments for primary Dupuytren's disease—a systematic review. J Hand Surg 2010;35B:623–6 [DOI] [PubMed] [Google Scholar]
- 2.Zhang AY, Cutrin CM, Hentz VR. Flexor tendon rupture after collagenase injection for Dupuytren's contracture: case report. J Hand Surg 2011;36A:1323–5 [DOI] [PubMed] [Google Scholar]
- 3.Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Eng J Med 2009;361:968–79 [DOI] [PubMed] [Google Scholar]


