Abstract
An 83-year-old woman developed pituitary metastasis while being treated for metastatic breast cancer. She presented with visual disturbance and headache followed by thirst, nocturia and polyuria. A visual field defect was present. MRI revealed a sellar mass consistent with metastasis to the pituitary gland. She was successfully treated with radiotherapy to the sella and had improvement of her visual symptoms and visual field defect. She then required ongoing treatment for diabetes insipidus. Her symptoms had not shown any sign of recurring up to 9 months after treatment. Pituitary metastases are rare but should be suspected in patients with metastatic cancer who present with features similar to those seen here. With improvements in survival in metastatic breast cancer, pituitary metastases may be seen more commonly and active local treatment is warranted given the possibility of resolution of symptoms related to the pituitary metastases.
Background
Even though metastases to the pituitary gland are rare, this case illustrates that early detection provides a chance to initiate treatment which can dramatically improve the symptoms related to this complication. As survival improves in metastatic breast cancer, this complication may be seen more frequently.
Case presentation
An 83-year-old woman who was being followed up for management of her metastatic breast cancer presented with decreasing vision in the left eye, headache and increasing thirst and polyuria progressing over a 2-month period.
She had originally been diagnosed with an invasive ductal carcinoma of the right breast in 1996, 16 years previously. At that time she underwent wide local excision followed by adjuvant radiotherapy. The tumour was a 15 mm, grade 2 invasive ductal carcinoma. There was no lymph node involvement. It was strongly positive for oestrogen receptors as well as progesterone receptors. She then had endocrine therapy with tamoxifen for 2 years. Tamoxifen was ceased 2 years later due to vaginal bleeding.
She had relapse of breast cancer 12 years later, in 2008, when she was diagnosed with bilateral small volume lung metastases. She was given letrozole with good response and had a 3-year period of good disease control.
There was further progression of metastatic breast cancer in 2011 when she was diagnosed with bone metastases in the skull and a subcutaneous nodule in the right supraclavicular fossa. She was treated with radiotherapy to both sites and was switched to exemestane with a good response.
She progressed well until October 2012 when she presented to hospital after a syncopal event while attending a concert. A non-contrast CT of the brain was performed which revealed an incidental 8 mm×16 mm×8 mm mass with some calcifications which appeared to arise from the sella. Just after this, prior to her oncology review in November 2012, she had a bone scan which showed progression of bone metastases. At this review she also described a history of decreasing vision in the left eye over the previous few months along with increasing thirst and urinary frequency over the previous few weeks.
On referral to an ophthalmologist, examination revealed decreased visual acuity on the left, measured at 6/24. Vision in the right eye was 6/6. The lenses were normal and the left macula had a normal appearance on funduscopy as well as optical coherence topography analysis. A visual field defect was present in the left eye which was demonstrated on a central 24-2 threshold test.
With the combination of visual changes, polyuria and polydipsia, along with the prior incidental finding of the mass in the sella on CT, MRI was performed which revealed a T2 hyperintense mass arising from the sella compressing the optic chiasm (figure 1). The features of the mass on MRI were consistent with a metastatic deposit and showed enhancement with gadolinium (figure 2). This irregular pituitary mass was new compared to MRI from 2011 (figure 3).
Figure 1.
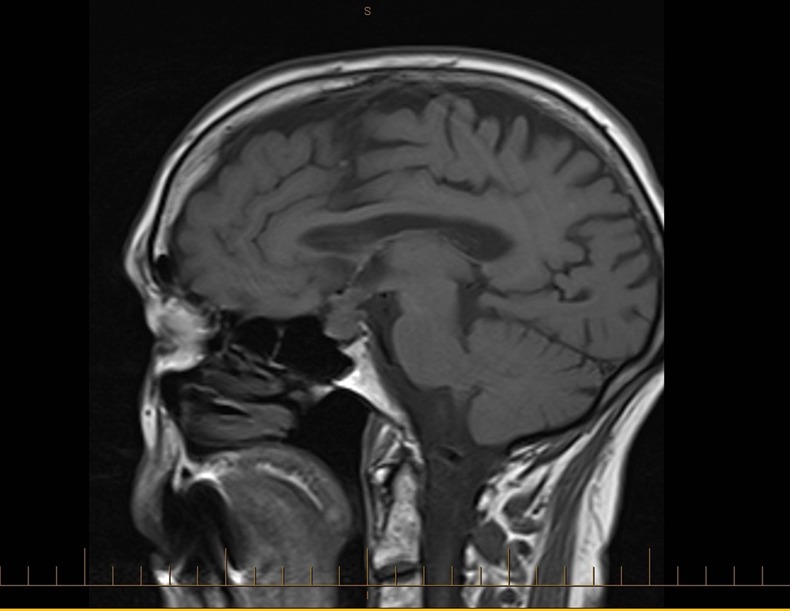
Sagittal T1 MRI from November 2012 showing the development of an irregular pituitary mass with thickened pituitary stalk.
Figure 2.
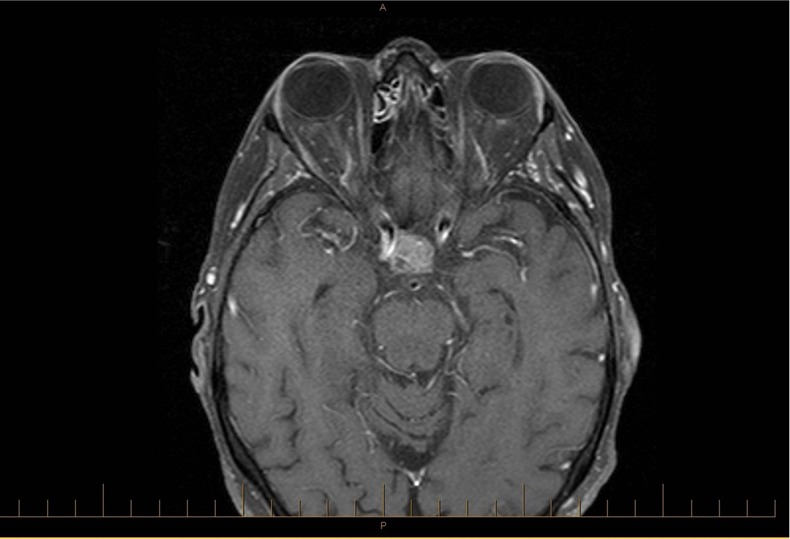
Axial T1 MRI postgadolinium from November 2012 showing enhancement of the pituitary mass consistent with pituitary metastasis.
Figure 3.
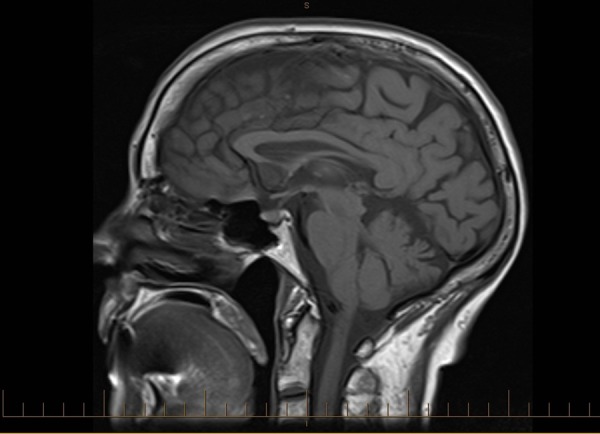
Sagittal T1 MRI from January 2011, prior to development of the pituitary mass.
Pituitary function tests revealed a raised prolactin level of 2115 mIU/L (normal <500). Thyroid function and adrenocorticotropic hormone levels were normal. A random serum cortisol was 287 nmol/L (normal 140–700 nmol/L). Her serum sodium was normal at 142 mmol/L (normal 135–145 mmol/L).
Treatment
After diagnosis of the pituitary metastasis along with progression of bone metastases, her systemic treatment was changed to tamoxifen and she was given dexamethasone 4 mg daily. She was referred for radiotherapy to the pituitary fossa.
She was treated with radiotherapy to the pituitary fossa and received a total of 30 Gy in 10 fractions to the pituitary fossa (figure 4). This resulted in a very significant improvement in her visual fields as well as visual acuity, and she was achieving 6/9 in the left eye at review in February 2013. Her headaches also resolved following radiotherapy.
Figure 4.
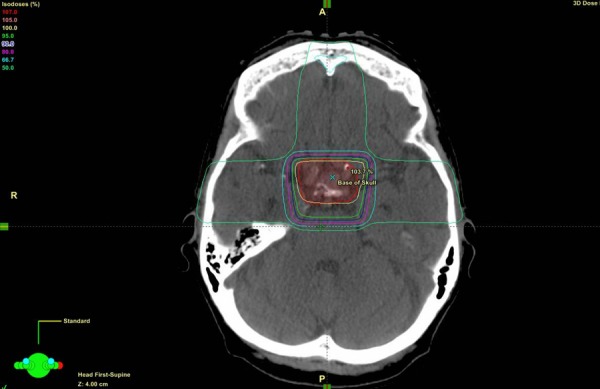
Transverse view of radiotherapy treatment plan.
Concurrently, she was treated for diabetes insipidus and was given desmopressin 10 μg intranasally daily. This improved her thirst and was titrated up to 20 μg daily however she then developed hyponatraemia with the sodium level falling to 130 mmol/L. With fluid restriction and reduction in the desmopressin dose back to 10 μg her sodium level improved to 140 mmol/L with continued good control of her symptoms.
Outcome and follow-up
Following completion of radiotherapy to the pituitary fossa there was resolution of the headaches and visual problems which continued to remain well controlled at follow-up 10 months later.
The diabetes insipidus remained well controlled and required ongoing treatment with desmopressin. Her steroid dose was weaned over the next few months and she was switched from dexamethasone to prednisolone. At 10 months after the initial diagnosis of pituitary metastases she remained on prednisolone 5 mg daily.
Her prolactin level also decreased over this time and stabilised close to 1000 mIU/L.
A follow-up MRI of the brain in March 2013, 5 months after the diagnosis of pituitary metastasis, showed significant improvement in the pituitary gland with only residual mild thickening of the pituitary stalk (figure 5).
Figure 5.
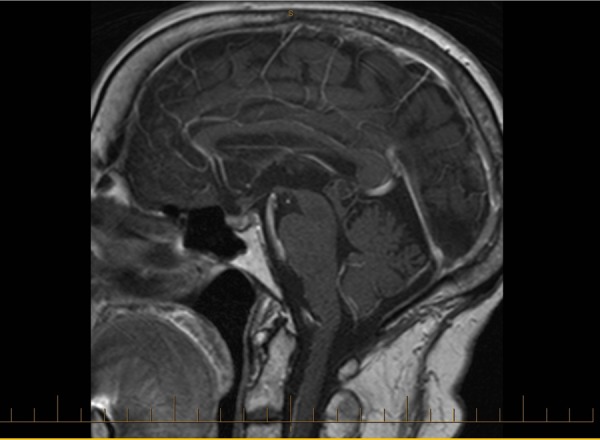
Sagittal T1 MRI with gadolinium from March 2013 post-treatment with radiotherapy showing reduction in the size of the pituitary mass.
Discussion
Background
Metastases to the pituitary gland are a rarer cause of pituitary masses. Pituitary adenomas are the most common cause of pituitary masses accounting for up to 10% of all intracranial neoplasms.1 Metastases to the pituitary gland account for about 1% of pituitary tumours as found in a large autopsy series.2 In one case series which included an autopsy series, the incidence of pituitary metastases was 3.6% while the incidence of pituitary adenomas was only 1.8% in the same group.3 This could possibly indicate that in the presence of known cancer, pituitary metastases could be more common than previously thought, given that in general, in a non-metastatic setting, pituitary adenomas account for about 84% of pituitary tumours.4
Various cancers can metastasise to the pituitary gland but by far the most common cancers to metastasise to the pituitary are from breast and lung origin.5 In one case series of 36 patients with symptomatic pituitary metastases, metastases from breast cancer accounted for 33% of pituitary metastases and lung accounted for 36%.6 The higher frequency of metastases from these sites may be related to the fact that these are among the most prevalent cancers across both sexes.7 Given that breast cancer is a common cause of metastases to the pituitary gland, it has been hypothesised by some authors that there could be a hormonal relationship for the increased frequency of breast metastases to the pituitary.8 Despite breast and lung cancers being the most common causes of pituitary metastases, there does not however appear to be a significant sex predominance with pituitary metastases6
Presentation
The presentation of pituitary metastases can vary and in fact most pituitary metastases may be clinically silent with one series showing that only 7% were symptomatic.9 When they are symptomatic, the most common presentations are with diabetes insipidus, anterior hypopituitarism, retroorbital pain/headache, visual defects and ophthalmoplegia.6 Interestingly, the presence of diabetes insipidus is much more common in pituitary metastases than in pituitary adenoma. As diabetes insipidus is such a common feature of pituitary metastases, it can be useful in differentiating pituitary adenoma from pituitary metastases. In some cases, diabetes insipidus can be the presenting feature of metastatic cancer.10
The patient in this case presentation presented with diabetes insipidus along with visual defects which fits the patterns described in the literature.
Treatment
The management of pituitary and brain metastases in general has improved over the past number of years. Earlier case series of pituitary metastases showed relatively poor survival with one study showing a median survival of 180 days.6 Given that breast cancer is one of the most common causes of pituitary metastases and that many options now exist to treat metastatic breast cancer, a more active approach to treatment, especially in oligometastatic disease seems to be appropriate in many cases now.11 The treatment modalities for pituitary metastases include surgical resection, three dimensional conformal radiotherapy and stereotactic radiotherapy. Systemic treatment with endocrine therapy or chemotherapy can also be used.
There is good evidence that local treatment directed at the pituitary metastases can provide excellent relief of symptoms associated with pituitary masses. Morita et al6 showed in their case series review that local control of tumour improved survival and significantly improved disabling symptoms associated with pituitary metastases including painful ophthalmoplegia and visual field defects.
The role for surgery in the setting of metastatic disease seems to be mainly in establishing the diagnosis. Surgery seems to be recommended when a symptomatic mass is present and the diagnosis is uncertain and when obtaining tissue would be useful in establishing the diagnosis in patients with limited metastatic disease.6 12 13 Diagnostic surgery, to guide treatment, has been recommended in the setting of diabetes insipidus in patients with known malignancy and a thickened pituitary stalk on MRI.14 Chandler et al15 recommend that when pituitary metastases are detected by frozen sections that pituitary surgery be terminated and the patient should proceed to other treatment modalities.
When metastases are likely, radiotherapy or γ knife radiosurgery are good options for relief of symptoms. In a case series of seven patients who had pituitary metastases treated with γ knife radiosurgery, resolution of symptoms was achieved in five patients. Interestingly, the survival varied between 0.3 and 42 months and seems more related to the extent of disease elsewhere than to the presence of the pituitary metastases.12
Piedra et al16 also showed a good improvement in the symptoms of diabetes insipidus by using γ knife radiosurgery and also showed that there was minimal effect to the nearby visual apparatus and no pituitary failure.
Pituitary adenoma versus pituitary metastases
An important point is to be able to differentiate between pituitary adenoma and pituitary metastases. In our case the prolactin level was found to be significantly elevated, which raised the question of whether a pituitary adenoma should be considered.
Generally a significantly elevated prolactin level is seen with adenomas however, any hypothalamic-pituitary mass lesion compressing the pituitary stalk can cause elevation of the prolactin level (disconnection hyperprolactinaemia).17
The hyperprolactinaemia seen in our case was most consistent with stalk compression from metastatic disease. Prolactin levels greater than 5000 mIU/L would be highly suggestive of primary pituitary adenoma whereas lower prolactin levels between 2000 and 4000 mIU/L are consistent with stalk compression.18 This was evident in our case where the initial prolactin level was 2115 mIU/L.
The MRI findings are useful in trying to differentiate the cause of the pituitary mass lesion. Pituitary metastases are usually isointense on T1-weighted images with a usually high intensity on T2-weighted images. This is in contrast to adenomas which tend to be isointense on T1 and T2 although these findings may not be highly specific. There is also homogenous enhancement with gadolinium.13
Clinically, the rate of progression is usually much faster for pituitary metastases than for adenomas. Pituitary metastases are also likely to invade the surrounding skull base, including the cavernous sinus and are much more likely to cause ophthalmoplegia.15
Conclusions
Overall, although the pituitary gland is a less common site of metastatic disease, it needs to be considered as a potential for metastases. This is especially true in patients with breast or lung cancer who present with diabetes insipidus, visual changes or headache. The presence of diabetes insipidus in particular should raise the concern for a pituitary metastasis.
Prolactin levels can be raised due to metastatic disease however as in this case they tend not to be as high as with adenoma. Pituitary stalk compression is the likely mechanism. MRI is essential in establishing the diagnosis.
Treatment can include surgery, radiotherapy (limited field radiotherapy and stereotactic, γ knife radiosurgery. Patients may need ongoing monitoring of pituitary function and treatment for ongoing pituitary hormone deficiency as was the case for this patient.
Metastases to the pituitary gland from breast and lung primaries may indicate more advanced disease but despite this, local treatment nearly always needs to be considered to provide improvement in symptoms. In this case, despite the progression of disease to the pituitary gland, treatment with localised radiotherapy proved very beneficial with resolution of the visual problems as well as headaches.
Overall, as survival in metastatic breast cancer continues to improve,19 20 the incidence of pituitary metastases is likely to increase. Therefore it is important to think about it in the setting of diabetes insipidus, headache or visual disturbance and even though treatment with radiotherapy may not improve life expectancy, this case shows that it can dramatically improve the symptoms caused by the pituitary metastases.
Learning points.
Pituitary metastases are rare but need to be considered with a presentation with diabetes insipidus, headache or visual disturbance in the setting of metastatic breast and lung cancer particularly.
Prolactin levels can help differentiate pituitary adenoma from pituitary metastases with prolactin levels between 2000 and 4000 mIU/L consistent with stalk compression and levels above 5000 mIU/L much more consistent with adenoma.
Active local treatment with radiotherapy can provide excellent improvement in the patient's symptoms.
Acknowledgments
The authors would like to thank Dr Tim Haymet, ophthalmologist.
Footnotes
Contributors: All of the authors had significant contributions of supporting material to the manuscript. JFG wrote the manuscript and corresponded regularly with all of the coauthors to provide insights and contributions relevant to their areas of expertise. The coauthors also provided support material in the form of radiotherapy treatment plans and results to describe the outcome for the patient in the manuscript. All of the authors were involved in the final editing of the case report and approved the final submission.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am 1999;28:81–117 vi [DOI] [PubMed] [Google Scholar]
- 2.Kovacs K. Metastatic cancer of the pituitary gland. Oncology 1973;27:533–42 [DOI] [PubMed] [Google Scholar]
- 3.Max MB, Deck MD, Rottenberg DA. Pituitary metastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology 1981;31:998–1002 [DOI] [PubMed] [Google Scholar]
- 4.Saeger W, Lüdecke DK, Buchfelder M, et al. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 2007;156:203–16 [DOI] [PubMed] [Google Scholar]
- 5.Asa SL. Practical pituitary pathology: what does the pathologist need to know? Arch Pathol Lab Med 2008;132:1231–40. [DOI] [PubMed] [Google Scholar]
- 6.Morita A, Meyer FB, Laws ER., Jr. Symptomatic pituitary metastases. J Neurosurg 1998;89:69–73 [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin 2013;63:11–30 [DOI] [PubMed] [Google Scholar]
- 8.Fasset D, Couldwell W. Metastases to the pituitary gland. Neurosurg Focus 2004;16:E8. [PubMed] [Google Scholar]
- 9.Teears RJ, Silverman EM. Clinicopathologic review of 88 cases of carcinoma metastatic to the pituitary gland. Cancer 1975;36:216–20 [DOI] [PubMed] [Google Scholar]
- 10.Kimmel DW, O'Neill BP. Systemic cancer presenting as diabetes insipidus. Clinical and radiographic features of 11 patients with a review of metastatic-induced diabetes insipidus. Cancer 1983;52:2355–8 [DOI] [PubMed] [Google Scholar]
- 11.Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst 2010;102:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwai Y, Yamanaka K, Honda Y, et al. Radiosurgery for pituitary metastases. Neurol Med Chir (Tokyo) 2004;44:112–16 discussion 117 [DOI] [PubMed] [Google Scholar]
- 13.Komninos J, Vlassopoulou V, Protopapa D, et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab 2004;89:574–80 [DOI] [PubMed] [Google Scholar]
- 14.Lin CS, Lin SH, Chiang YH, et al. Diabetes insipidus revealing an isolated pituitary stalk metastasis of breast cancer. Eur J Neurol 2007;14:e11–12 [DOI] [PubMed] [Google Scholar]
- 15.Chandler WF, Barkan AL. Treatment of pituitary tumors: a surgical perspective. Endocrinol Metab Clin North Am 2008;37:51–66 viii [DOI] [PubMed] [Google Scholar]
- 16.Piedra MP, Brown PD, Carpenter PC, et al. Resolution of diabetes insipidus following gamma knife surgery for a solitary metastasis to the pituitary stalk. Case report. J Neurosurg 2004;101:1053–6 [DOI] [PubMed] [Google Scholar]
- 17.Mancini T, Casanueva FF, Giustina A. Hyperprolactinemia and prolactinomas. Endocrinol Metab Clin North Am 2008;37:67–99 viii [DOI] [PubMed] [Google Scholar]
- 18.Levy A. Pituitary disease: presentation, diagnosis, and management. J Neurol Neurosurg Psychiatry 2004;75 (Suppl 3):iii47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol 2008;26:4891–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Trends in survival for patients with recurrent breast cancer diagnosed from 1974 through 2000. Cancer 2004;100:44–52 [DOI] [PubMed] [Google Scholar]


