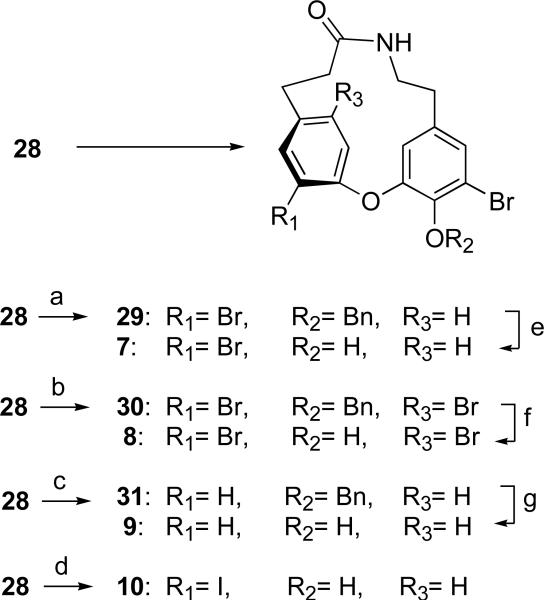
Scheme 5.
Preparation of C-aryl-substituted 14-membered ring analogs.
Key: (a) i. CuBr2 (0.8 equiv), tBuONO, CH3CN, 0 °C, 1h; ii. 27 in portions, 0 °C, 2h then warmed to rt; iii. 1N HCl, 66% (b) i. CuBr2 (10 eq), tBuONO, CH3CN, 0 °C, 1h; ii. 28 in portions, 0 °C, 2h then warmed to rt; iii. 1N HCl, 32%; (c) i. tBuONO, THF, 0 °C, 1h; ii. 28 in portions, 0 °C, 2h then warmed to rt; iii. 1N HCl, 39%; (d) i. H2SO4, AcOH, NaNO2, 0 °C, 0.5 h; ii. 28 in portions, 0 °C, 1 h then warmed to rt, 20 min; iii. KI, H2O, rt, 15 min then warmed to 70 °C, 15 min, 16%; (e) BBr3, –78 ° C, 1h, 91%; (f) TFA, rt, 24h, 70%; (g) TFA, rt, 24h, 75%.